
| A����a��3b��2c��/2 kJ?mol��1 | B����a��3b��2c��/6 kJ?mol��1 |
| C����a��3b��2c��/6 kJ?mol��1 | D����a��3b��2c��/3 kJ?mol��1 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
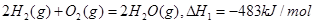 ��������Ȼ�ѧ����ʽ��
��������Ȼ�ѧ����ʽ��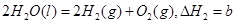 ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��| A���Ȼ�ѧ��Ӧ����ʽ�л�ѧ��������ʾ���Ӹ��� |
B���÷�Ӧ��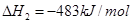 |
C��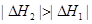 |
D��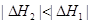 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________��
ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ________________ ________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������H<0ʱ����ʾ�÷�ӦΪ���ȷ�Ӧ |
| B����Ҫ���Ȳ��ܽ��еķ�Ӧ�����ȷ�Ӧ |
| C����Ӧ�ȵĴ�С�뷴Ӧ�������е������������������е������� |
| D��1molNaOH�ֱ��1molCH3COOH��1molHNO3��Ӧ�ų���������CH3COOH<HNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����10 NA������ת��ʱ���÷�Ӧ�ų�1300kJ������ |
| B����1 NA��ˮ����������ΪҺ��ʱ������1300kJ������ |
| C����2 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
| D����8 NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���
2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���| | H2(g) | Br2(g) | HBr(g) |
| ����/kJ��mol��1 | 436 | a | 369 |
 l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________��
l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com