

| | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | �� | �� | �� | ʵ��Ŀ�� |
| A | Ũ��ˮ | NaOH | ����ʳ��ˮ | ��ȡ���ռ����� |
| B | Ũ���� | MnO2 | ����ʳ��ˮ | ��ȡ���ռ����� |
| C | ϡ���� | Cu | ˮ | ��ȡ���ռ�һ������ |
| D | ϡ���� | Zn | ˮ | ��ȡ���ռ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
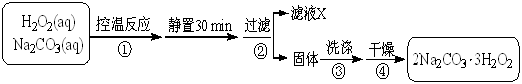
 2Na2CO3��3H2O2 (s) ��H < 0
2Na2CO3��3H2O2 (s) ��H < 0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
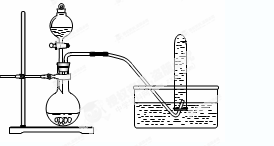
| A��ͭ��Ũ���ᷴӦ��NO2���ɲ�����ͼװ�� |
| B������������Ҵ���Һ���÷�Һ©�����з��� |
| C��ϡ�����п����Ӧ��ȡ������������������ͭ�Լӿ췴Ӧ���� |
| D������Na2CO3��Һ��NaHCO3��Һ���ֱ���������Һ�μӳ���ʯ��ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
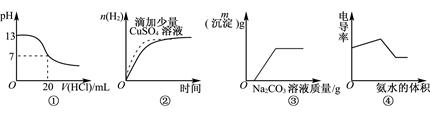
| A��ͼ�ٱ�ʾ25��ʱ��0.1mol��L��1����ζ�20mL 0.1mol��L��1NaOH��Һ |
| B��ͼ�ڱ�ʾ�����£�����п���������ĵ������Ũ�ȵ����ᷴӦ |
| C��ͼ�۱�ʾ��CaCl2������Ļ����Һ�еμ�Na2CO3��Һ |
| D��ͼ�ܱ�ʾ������ʹ�������Һ�е��백ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
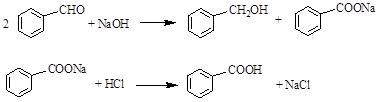

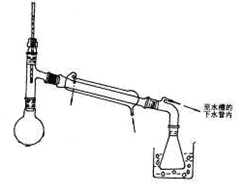
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
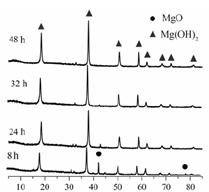
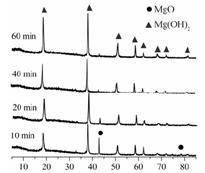


| LiOH | NaOH | KOH | Al(OH)3 | Mg(OH)2 | Ca(OH)2 | Ba(OH)2 |
| 924 | ���ֽ� | ���ֽ� | 140 | 258 | 390 | 700 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
�� ��H��===2Mn2����5
��H��===2Mn2����5 ��3H2O����ջش����⣺
��3H2O����ջش����⣺| A����ʽ�ζ���(50 mL) | B����ʽ�ζ���(50 mL) |
| C����Ͳ(10 mL) | D����ƿ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com