
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ������������������±���ʾ��
| ������ | NH4+��Na����Mg2�� |
| ������ | OH����NO3����SO42�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��13�֣�ijǿ������ҺX���ܺ���Ba2+��A13+��NH4+��Fe2+��Fe3+��CO32-��SO32-��SO42-��C1-��NO3-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飬ʵ��������£�����������Ϣ���ش��������⣺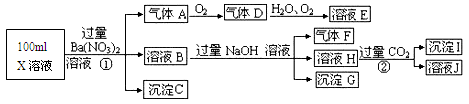
��1������FΪ____________��
��2�����������У���ҺX�г�H+��϶����е�������_______������ȷ���Ƿ��е�������____��
��3��д������A�����ӷ���ʽ��_________________��
��4��ͨ����������KClO�ڼ�������������G���Ʊ�һ�����͡���Ч�����ˮ������K2FeO4����д���Ʊ������е����ӷ���ʽ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

��ʾ���ڱ�ʵ�������£�Ni(��)���ܱ�������������صĻ�ԭ������MnO2
�ش��������⣺
��1����Ӧ���г��������������� ��������Ӧ�����ӷ���ʽΪ ���ڼӸ��������Һǰ����pH�ϵͣ��Գ��ӵ�Ӱ���� ��
��2����Ӧ�۵ķ�Ӧ����Ϊ �����˵õ��������У����˹�����п��� ��
��3����Ӧ���γɵij���Ҫ��ˮϴ����������Ƿ�ϴ�Ӹɾ��ķ����� ��
��4����Ӧ���в���ijɷֿ�����ZnCO3��xZn(OH)2ȡ�ɲٺ���˱�11.2g�����պ�ɵõ���Ʒ8.1g����x���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����һ������Fe�ۡ�Cu�ۡ�FeCl3��Һ��CuCl2��Һ�����ij�����г�ַ�Ӧ(�ٶ����������뷴Ӧ)���ж���������������н���������������ʵĴ�����������÷��ű�ʾ��
����Fe����ʣ�࣬�������в�������____________��
����FeCl3��ʣ�࣬�������в�������___________��
����CuCl2��ʣ�࣬�������л�������____________��
��2����һƿ��ɫ������Һ,���ܺ�NH4����K����Na ����Mg2����Ba2���� Al3���� Fe3����SO42����CO32����Cl����I���е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����pH��ֽ���飬������Һ�����ԣ�
��ȡ������Һ����������CCl4���������Ƶ���ˮ�������ã�CCl4����Ϻ�ɫ��
��ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о��������ɣ�
��ȡ���������ʼ��Ե���Һ����Na2CO3��Һ���а�ɫ�������ɣ�
�ݽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ�����ش�����Һ�п϶����ڵ������������� ���϶������ڵ��������� �� ��Ҫȷ���Ƿ���ڵ������ӵ�ʵ�鷽����____________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�й�FeSO4��ת����ϵ��ͼ��ʾ(������������ȥ)��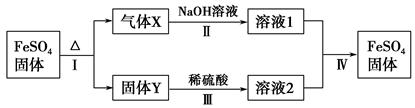
��֪����X�����ֻ�������ɣ���Xͨ��Ʒ����Һ����Һ��ɫ��ͨ��BaCl2��Һ��������ɫ������
��Y�Ǻ���ɫ�Ļ����
(1)����X�ijɷ���(�ѧʽ) ��
(2)��Ӧ��ķ�Ӧ��������(�����) ��
a���ֽⷴӦ b�����ֽⷴӦ
c���û���Ӧ d�����Ϸ�Ӧ
e��������ԭ��Ӧ
(3)��Һ2�н��������ӵļ��鷽���� ��
(4)������Ӧ��õ�16 g����Y������������Xǡ�ñ�0.4 L 1 mol��L��1 NaOH��Һ��ȫ���գ���Ӧ��������FeSO4�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij������Һ�п��ܺ���SO42-��SO32-��CO32-��HCO3-��NO3-��Cl����Br���е������ּ�һ�ֳ�������������(Mn��)���ֽ�������ʵ��(ÿ��ʵ�������Լ����������ģ�������ijЩ�ɷֿ���û�и���)��
��ش��������⣺
(1)����������ͼ��Ϣ��д�±�(����ȷ���IJ���)��
| | �϶����ڵ����� | �϶�û�е����� | ����D | |
| ��ѧʽ�����ӷ��� | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.�������(K2FeO4)�Ǽ��õ������������и�Ч���������ã�Ϊһ�����ͷ��ȸ�Ч�������������������������£�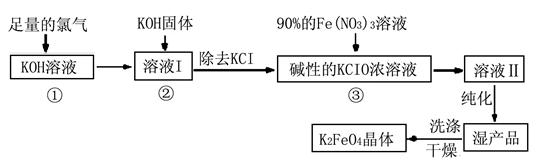
��ͬ���������⡣
��1��д����KOH��Һ��ͨ������Cl2������Ӧ�����ӷ���ʽ ��
��2������ҺI�м���KOH�����Ŀ���� (ѡ�����)��
| A��Ϊ��һ����Ӧ�ṩ���ԵĻ��� |
| B��ʹKClO3ת��ΪKClO |
| C������ҺI�й�����Cl2������Ӧ�����ɸ����KClO |
| D��KOH�����ܽ��ų��϶����������������߷�Ӧ���ʺ�KClO�Ĵ��� |
 3Zn+2K2FeO4+8H2O��
3Zn+2K2FeO4+8H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��ˮ�г�����һ������Cr2O72����CrO42�������ǻ�����༰��̬ϵͳ�����ܴ���˺���������д������÷��Ĺ�������Ϊ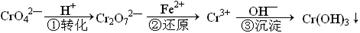
���еڢٲ�����ƽ�⣺2CrO42������ɫ��+2H+ Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O
��1����ƽ����ϵ��pH=2������Һ�� ɫ��
��2����˵���ڢٲ���Ӧ��ƽ��״̬���� ����ѡ���ţ�
| A��Cr2O72����CrO42����Ũ����ͬ | B��v��(Cr2O72��) ="2v" ��(CrO42��) |
| C����Һ����ɫ���� | D����Һ��pHֵ���� |
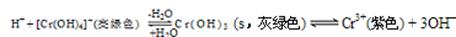
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��
��.��1��ClO2����KClO3��H2SO4���ڵ���������Na2SO3��Ӧ�Ƶá���÷�Ӧ�����������뻹ԭ��������ʵ���֮����________��
��.ʵ����Ҳ����NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ���Ʊ�ClO2�����������£�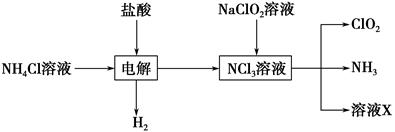
��2��д�����ʱ������Ӧ�Ļ�ѧ����ʽ��________________________________��
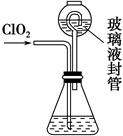
��3����ȥClO2�е�NH3��ѡ�õ��Լ���________��������ţ�
| A������ʳ��ˮ | B����ʯ�� |
| C��Ũ���� | D��ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com