
�й�FeSO4��ת����ϵ��ͼ��ʾ(������������ȥ)��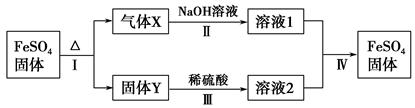
��֪����X�����ֻ�������ɣ���Xͨ��Ʒ����Һ����Һ��ɫ��ͨ��BaCl2��Һ��������ɫ������
��Y�Ǻ���ɫ�Ļ����
(1)����X�ijɷ���(�ѧʽ) ��
(2)��Ӧ��ķ�Ӧ��������(�����) ��
a���ֽⷴӦ b�����ֽⷴӦ
c���û���Ӧ d�����Ϸ�Ӧ
e��������ԭ��Ӧ
(3)��Һ2�н��������ӵļ��鷽���� ��
(4)������Ӧ��õ�16 g����Y������������Xǡ�ñ�0.4 L 1 mol��L��1 NaOH��Һ��ȫ���գ���Ӧ��������FeSO4�����ӷ���ʽ�� ��
 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ӷ���ʽ����д��ȷ���ǣ� ��
| A������ϡ���ᷴӦ��2Fe + 6H+ ��2Fe 3+ +3H 2�� |
| B��NaHCO3��Һ��NaOH��Һ��Ӧ�� OH�D + HCO3�D�� CO32�D + H2O |
| C���ƺ���ˮ��Ӧ Na��2H2O��Na+��2OH-��H2�� |
| D���Ȼ�����Һ�м�������İ�ˮ Al3+ + 4NH3��H2O ��AlO2�� + 4NH4++ 2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14��)����п�������ᡢ�´ɡ����¡�ҽҩ�����ӡ���ѧ�ȹ�ҵ����Ҫԭ�ϡ�����
��п��ƷΪԭ���Ʊ���������п�����������������£�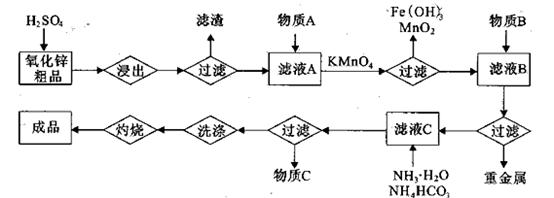
��1��.��������õ���������Һ�к���Zn2����SO42����������Fe2����Cu2���� ����Mn2����
����Mn2����
���ʡ�����A�������ǵ�����Һ��pH��5 4������A���ѡ��________��
| A��NH3.H2O | B��Na2CO3 | C��H2SO4 | D��ZnO |
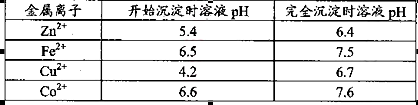
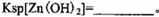 ��
��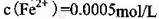 ������1
������1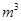 ����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���
����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���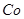 2���������û���Ӧ��ȥ��������B��_________��
2���������û���Ӧ��ȥ��������B��_________��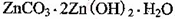 �����ɸó����Ļ�ѧ����ʽΪ________��
�����ɸó����Ļ�ѧ����ʽΪ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2���� ��
�� ��Cl����I����
��Cl����I���� ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ����Һ�п��ܴ�������Ag����Mg2����Cu2����Fe3����Na���еļ��֣�����д���пհף�
(1)�����κ�ʵ��Ϳ��Կ϶�ԭ��Һ�в����ڵ������� ��
(2)ȡ����ԭ��Һ���������ϡ���ᣬ�а�ɫ�������ɣ��ټ��������ϡ���ᣬ��������ʧ��˵��ԭ��Һ�п϶����ڵ������� ���йص����ӷ���ʽΪ ��
(3)ȡ(2)�е���Һ�����������ϡ��ˮ(NH3��H2O)�����ְ�ɫ������˵��ԭ��Һ�п϶��� ���йص����ӷ���ʽΪ ��
(4)ԭ��Һ���ܴ������ڵ������������е� ��
| A��Cl�� | B��NO3�� | C��CO32�� | D��OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ������������������±���ʾ��
| ������ | NH4+��Na����Mg2�� |
| ������ | OH����NO3����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ������Һ��Ҫȷ���Ƿ����������ӣ�H+��K����Mg2����Al3����Fe2����Ba2����NO3����SO42����Cl����I����HCO3����ȡ����Һʵ�����£�
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ���ɫ |
| (2)ȡ��������Һ����Ũ������CuƬ��ŨH2SO4������ | ����ɫ����������������������Ա�ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����HNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ�к���Fe2����Al3����Ag����Cu2����Ϊ�ֱ�õ�����һ�ֽ��������ӵij�����ɲ�ȡ��ͨ��H2S���壬��ͨ��CO2���壬�ۼ������ᣬ�ܼ�������������Һ�ĸ����裬ÿ��ͨ�������Լ�����������ÿ�ζ��ѳ������˳������������ȷ˳����(����)��
| A���ۢ٢ܢ� | B���٢ۢܢ� | C���ܢڢ٢� | D���ܢڢۢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com