

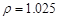 g/cm3 )����ȡ��36.5% (
g/cm3 )����ȡ��36.5% (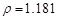 g/cm3 )������ mL
g/cm3 )������ mL
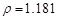 g/cm3 )���������ΪX
g/cm3 )���������ΪX 

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Թ�ȡ���Լ�ƿ�е�NaOH��Һ������ȡ�����࣬Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�� |
| B��������Ӧʵ���ķ�Һ���ȵ���ˮ���У�����ˮ������ˮ�� |
| C�����Թ��еμ�Һ��ʱ����ͷ�ιܱ�������Թ��ڱڣ�����Һ�彦�� |
| D����Ũ��������һ�����ʵ���Ũ�ȵ�ϡ����ʱ��Ũ��������ˮ��Ӧ��ȴ�����²���ת�Ƶ�����ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
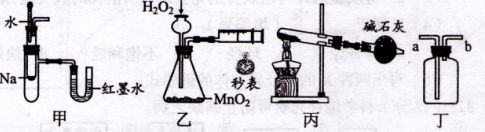
| A��װ�ü�������֤��Ӧ����ЧӦ |
| B��װ���ҿɶ����ⶨ��ѧ��Ӧ������ |
| C��װ�ñ�������ʵ������NH4ClΪԭ���Ʊ�����NH3 |
| D��װ�ö�a�ڽ������ռ�NH3��C12������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 12Na2CrO4+3Fe2O3+7KCl+12H2O��
12Na2CrO4+3Fe2O3+7KCl+12H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ʊ����ᶡ�������������������ᣬ�ٵμӼ���Ũ���ᣬ��ˮԡ���� |
B�����������Һ���Ƿ��������̪����ͼ��ʾ����ֽ�ϲ�����ʵ��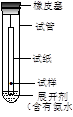 |
| C������ȩ��������CuSO4��Һ��NaOH��Һ��1mL����������ȩ��Һ����� |
| D���Ƚϱ��ӡ����ᡢ̼������ԣ�����ʹ��Ӧ����������ͨ�뱽������Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

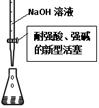
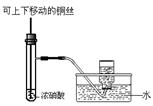
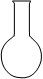
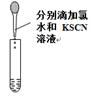
| A���â���ȡ15.00 mL NaOH��Һ |
| B���â��Ʊ����ռ�����NO2���� |
| C���â���ʾ����������1L 0.1000mol/LNaCl��Һ |
| D���âܽ���Fe2+�ļ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com