
25 �桢101 kPa�£�̼������������������ǵ�ȼ����������393.5 kJ��mol-1��285.8 kJ��mol-1��890.3 kJ��mol-1��2 800 kJ��mol-1���������Ȼ�ѧ����ʽ��ȷ���� �� ��
A��C(s)+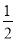 O2(g)====CO(g);��H=-393.5 kJ��mol-1
O2(g)====CO(g);��H=-393.5 kJ��mol-1
B��2H2(g)+O2(g)====2H2O(g);��H=+571.6 kJ��mol-1
C��CH4(g)+2O2(g)====CO2(g)+2H2O(g);��H=-890.3 kJ��mol-1
D�� C6H12O6(s)+3O2(g)====3CO2(g)+3H2O(l);��H=-1 400 kJ��mol-1
C6H12O6(s)+3O2(g)====3CO2(g)+3H2O(l);��H=-1 400 kJ��mol-1
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶���ѧ�ڵ�һ���¿��Ŀƻ�ѧ���������棩 ���ͣ�ѡ����
����ϡ�����Ũ����ȼ��ְ�ȫ�ķ�����
A����ȡ�������Թ��м��뼸ƬͭƬ
B���ò�������պ����������ֽ��
C����ȡ�������Թ��еμ�NaOH��Һ
D����ȡ�������Թ��еμ�BaCl2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�����и�һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣�ʵ����
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������200 mL 1.0 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У�

�ٲ�����������ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
��1����������ϡ����ʱ����ȱ�ٵ�������________________________(д��������)��
��2����ǩ��ʾŨ��������ʵ���Ũ��Ϊ___________________________
��3������200 mL 1.0 mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ_______mL������������С�����1λ������ȡ����ʱӦѡ��_______������Ͳ��
A��10 mL B��50 mL
C��100 mL D��200 mL
��4�����ݼ���������������ʵ�������
������Ͳȡ�������������Ũ���
������Ͳ�м�����������ˮ�����ò��������裻
��������ϡ�ͺ����Һת������ƿ�У�
��Ȼ������ˮ�ز�����ע������ƿֱ���̶��ߣ�
�ݰ�����ƿ�Ǹǽ������µߵ�ҡ�ȡ�
����Ϊ����ʵ���д���IJ�����______________________________������ţ�
��5�������ⶨ��ijͬѧ���Ƶ�ϡ����Ũ��ƫ�ߣ�����ܵ�ԭ����_______������ţ�
������Ͳ��ȡŨ����ʱ�����ӿ̶���
������ƿ������ˮϴ�Ӻ�δ������
��ϴ���ձ��ڱں�ϴ��Һ��ȥ
��ת����Һʱ��������������Һ����
�ݶ���ʱ����������ƿ�̶���
���ݡ�ҡ�Ⱥ�����Һ�İ�Һ����ڿ̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫��ݸ�и߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��1����(N2H4)�ǻ����������ȼ�ϣ�����N2O4��Ӧʱ��N2O4Ϊ�����������ɵ�����ˮ������
��֪��N2(g)��2O2(g)===N2O4(g) ��H����8.7 kJ/mol��N2H4(g)��O2(g)===N2(g)��2H2O(g)��H����534.0 kJ/mol�� ���ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ
��2����CO2�������ϳ�CH3OCH3(����)�ǽ����ԴΣ�����о�����֮һ��
��֪��CO(g)��2H2(g)  CH3OH(g) ��H����90.7 kJ��mol��1
CH3OH(g) ��H����90.7 kJ��mol��1
2CH3OH(g)  CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1
CH3OCH3(g)��H2O(g) ��H����23.5 kJ��mol��1
CO(g)��H2O(g)  CO2(g)��H2(g) ��H����41.2 kJ��mol��1
CO2(g)��H2(g) ��H����41.2 kJ��mol��1
��CO2�������ϳ�CH3OCH3(g)���Ȼ�ѧ����ʽΪ
______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫��ݸ�и߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�����Һ��ͬʱ��ʼ��Ӧ�����ֻ����������
A��20��ʱ 5mL 0.05mol��L-1 Na2S2O3��Һ��5mL 0.1mol��L-1������
B��20��ʱ 50mL 0.1mol��L-1 Na2S2O3��Һ��50mL 0.1mol��L-1������
C��10��ʱ 5mL 0.05mol��L-1 Na2S2O3��Һ��5mL 0.1mol��L-1������
D��10��ʱ 5mL 0.1mol��L-1 Na2S2O3��Һ��5mL 0.1mol��L-1������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ɽ��ʡ��һ��ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�������
��6�֣���CuSO4��H2SO4�Ļ����Һ200mL������CuSO4���ʵ���Ũ��Ϊ1mol��L-1, H2SO4���ʵ���Ũ��Ϊ0��5mol��L-1,���ô���Һ���0��2mol��L-1 CuSO4��2mol��L-1 H2SO4�Ļ����Һ,����:
��1��������Һ������Ƕ��ٺ���?
��2��������ܶ�Ϊ1��84g��cm-3��98%��Ũ������ٺ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ɽ��ʡ��һ��ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�鷽������У����е��� �� ��
A����ϡ�������ˣ���ȥ����ͭ���е�����þ�ۺ�����
B������ȡ�ķ����������ͺ�ú��
C�����ܽ⡢���˵ķ�������KNO3��NaCl����Ļ����
D����H2��O2�Ļ������ͨ�����ȵ�����ͭ���Գ�ȥ���е�H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ɽ��ʡ�߶�10���¿���ѧ�Ծ��������棩 ���ͣ������
���ܱ������У���һ����ʼŨ�ȵ��(Xe)��F2��Ӧ���ɵõ����ַ����������������ƽ����ϵ�ڵķ�ѹ�뷴Ӧ�¶ȵĹ�ϵ����ͼ��ʾ(��֪����ķ�ѹ֮�ȵ������ʵ���֮��)��
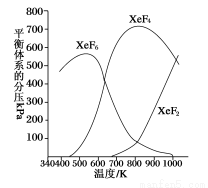
��1��420 Kʱ��������Ӧ�Ļ�ѧ����ʽΪ__________________________������Ӧ������1 mol Xe����ת�Ƶ���____________mol��
��2��600��800 Kʱ���ᷢ����Ӧ��XeF6(g) XeF4(g)��F2(g)���䷴Ӧ�Ȧ�H________0(�����������������)��������_________________________________________________����3��900 Kʱ�������д��ڵ��������__________________________________���ѧʽ����
XeF4(g)��F2(g)���䷴Ӧ�Ȧ�H________0(�����������������)��������_________________________________________________����3��900 Kʱ�������д��ڵ��������__________________________________���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��ɽ����ѧ���е�һѧ�ڸ߶���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ϳɰ��ķ�ӦΪ��3H2 + N2 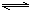 2NH3���䷴Ӧ�����ʿɷֱ��ʾΪv(H2)��v(N2)��v(NH3)(��λΪmol��L��1��s��1)�������й�ϵ��ȷ���� �� ��
2NH3���䷴Ӧ�����ʿɷֱ��ʾΪv(H2)��v(N2)��v(NH3)(��λΪmol��L��1��s��1)�������й�ϵ��ȷ���� �� ��
A��v(H2)��v(N2)= v(NH3) B��v(H2)��3 v(N2)
C��v(N2)��2v(NH3) D��v(NH3)��3/2v(H2)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com