
�ס��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ��������ʵ�����̣�


ʵ���У������Ƶĵİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ����Ӧ��Ϻ�ɫ������ͭת��Ϊ��ɫ��ͭ����ͼA��B��CΪ�ס�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��
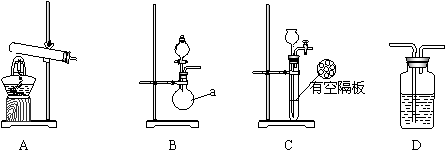

��С���ã���Ӧǰ����ͭ������m1g������ͭ��Ӧ��ʣ����������m2g�����ɰ����ڱ�״���µ����V1L��
��С���ã�ϴ��ǰװ��D������m3g��ϴ����װ��D������m4g�����ɰ����ڱ�״���µ����V2L��
��ش��������⣺
��1�������a������ ��
��2�����Aװ�������ԵIJ����� ��������
��3���ס�����С��ѡ���˲�ͬ�ķ�����ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��Ŀո��С�
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
| ��С�� | A | �������ơ����ᡢ����� | ��Ӧ�Ļ�ѧ����ʽΪ�١� ���������������������� �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� ���������� �� |
��4����С������������ݼ�����������е������ԭ�Ӹ���֮��Ϊ ��
��5����С������������ݼ�����������е������ԭ�Ӹ���������С������ֵ����ԭ���� ��Ϊ�ˣ���С����ԭ��ʵ��Ļ�����������һ��װ��ijҩƷ��ʵ������������ʵ�顣����ʵ��ǰ���ҩƷ�������仯�����ɰ�����������ó��˺�����ʵ��������ҩƷ�������� ��
�𰸣���Բ����ƿ��2�֣�
�����ӵ��ܣ������ܲ���ˮ�У������¹أ����ܿ������ݲ�����ֹͣ���ȣ���������ˮ�������γ�һ���ȶ���ˮ����3�֣�
�Ǣ�(NH4)2SO4+Ca(OH)2 2NH3��+2H2O+CaSO4��2�֣� ��B��2�֣�
2NH3��+2H2O+CaSO4��2�֣� ��B��2�֣�
�������������ڰ�ˮ����ȣ�����������Ũ�ȣ�ʹNH3+H2O NH3��H2O
NH3��H2O NH4��+OH�����淴Ӧ�����ƶ����ӿ찱���ݳ���2�֣�
NH4��+OH�����淴Ӧ�����ƶ����ӿ찱���ݳ���2�֣�
��5V1:7(m1��m2) ��2�֣�
��Ũ����������δ��Ӧ�İ������Ӷ�ʹ����İ��ĺ���ƫ�ߣ�2�֣�
��ʯ�ң��������ơ������Ƶȣ���2�֣�


 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���У�����ȡ�ķ��뷽�����Ӧԭ������ȷ����
| ѡ�� | Ŀ�� | ���뷽�� | ԭ�� |
| A | ��������ˮ�ĵ� | �Ҵ���ȡ | �����Ҵ��е��ܽ�Ƚϴ� |
| B | ���������������Ҵ� | ��Һ | �����������Ҵ����ܶȲ�ͬ |
| C | ��ȥKNO3�����л��ӵ�NaCl | �ؽᾧ | NaCl��ˮ�е��ܽ�Ⱥܴ� |
| D | ��ȥ�����е����� | ���� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ������þ���ǽ�þ��������������ȴ������Ϊȴ������ǡ� ���� ��
�ٿ��� ��CO2 ��Ar ��H2 ��N2
A���٢� B���ڢ� C���ۢ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������ Ҫ��Ӧ�á�
Ҫ��Ӧ�á�
(1)����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)��10C(s)=6CaO(s)��P4(s)��10CO(g) ��H1=��3359.26 kJ��mol��1
CaO(s)��SiO2(s)=CaSiO3(s) ��H2=��89.61 kJ��mol��1
2Ca3(PO4)2(s)��6SiO2(s)��10C(s)=6CaSiO3(s)��P4(s)��10CO(g) ��H3
���H3= kJ��mol��1��
(2)�����ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P4��60CuSO4��96H2O=20Cu3P��24H3PO4��60H2SO4
60molCuSO4�������������ʵ����� ��
(3)����Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH �Ĺ�ϵ����ͼ��ʾ��
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������ ��pH=8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ ��
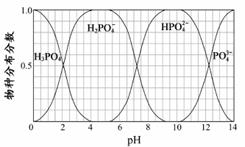
��Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����
(�����ӷ���ʽ��ʾ)��
(4)�Ļ�������������( )�뼾���Ĵ�(
)�뼾���Ĵ�(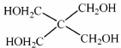 )�����ʵ���֮��2:1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��
)�����ʵ���֮��2:1 ��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X �ĺ˴Ź�����������ͼ��ʾ��
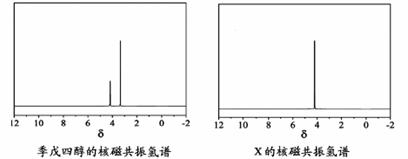
������������ (�ѧʽ)��
��X�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PH3��һ����ɫ�綾���壬����ӽṹ��NH3���ƣ���P−H�����ܱ�N−H�����ܵ͡������жϴ������
A��PH3���ӳ�������
B�� PH3�����Ǽ��Է���
C��PH3�е����NH3�е㣬��ΪP-H�����ܵ�
D��PH3�����ȶ��Ե���NH3���ӣ���ΪN-H�����ܸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NaCl��һ�ֻ���ԭ�ϣ������Ʊ�һϵ�����ʣ���ͼ4��������˵����ȷ����
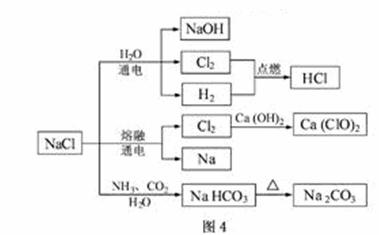
A.25�棬NaHCO3��ˮ�е��ܽ�ȱ�Na2CO3�Ĵ�
B.ʯ������Cl2�ķ�Ӧ�У�Cl2���������������ǻ�ԭ��
C.�����¸����Cl2���ø�ƿ���棬����Cl2��������Ӧ
D.ͼ4��ʾת����Ӧ����������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪�������ԣ�
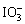 ��Fe3����I2����ԭ�ԣ�
��Fe3����I2����ԭ�ԣ� ��I����
��I����
3I2��6OH��
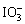 ��5I����3H2O��
��5I����3H2O��
KI��I2 KI3
KI3
��1��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ������_________���û�ѧʽ��ʾ����CCl4�����Ϻ�ɫ��������___________________���õ���ʽ��ʾ����
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ___________________________��______________________________________��
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��
д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_____________________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ�______����ǡ�������˵������________________________________________��
��3��Ϊ����ӵ��Σ�����KI�����ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ�������___________________��
A��Na2S2O3 B��AlCl3 C ��Na2CO3 D��NaNO2
��4���Ժ�Fe2���϶��ʳ��(���費��Fe3��)����ѡ��KI��Ϊ�ӵ���������ʵ�鷽��������üӵ����е�Fe2����__________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ���γɵij����ǽ������嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ����D������E��D��������ǿ�ᣬҲ������ǿ�E������������ȼ�ղ����̼�������G��G�ڴ������ܵ���������γɡ�E����������������Һ���յõ���ɫ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��������ƵĽṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��ش��������⣺
��1����ɵ���A��Ԫ��λ�����ڱ��е� ���ڣ��� �塣
��2��B������������Һ��Ӧ�Ļ�ѧ����ʽΪ�� ��
��3��G�������������������·�Ӧ����������ɱ�����������ȡ��÷�Ӧ����������Ϊ ��������2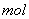 ��������ʱ��ת�Ƶ���
��������ʱ��ת�Ƶ��� 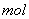 ��
��
��4����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ�� ��
��5��H����Һ��ϡ���ᷴӦ����������Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����ñ�ɫ���ⶨ������������淋Ĵ��ȵ�˵����ȷ����(����)
A�����Ʊ�ɫ��ʹ�õĵζ����Ǽ�ʽ�ζ���
B���ڲ�ͬ����ı�ɫ����Fe3��Ũ�Ȳ��Խ�ⶨ����������淋Ĵ������ԽС
C�����Ʊ�ɫ��ʱ������һ�����������Ŀ��������Fe3����ˮ��
D���ô����Ʒ��Һ���ɫ�ױȽϣ�����ȷȷ����Ʒ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com