
| ʵ�鷽�� | ������ |
| ʵ�鷽�� | ������ |
| ȡ������������ƿ�У���������3 mol?L-1 H2SO4�����ϴ������ܵ���Ƥ���������������嵼��������ͨ��ʢ������KMnO4��Һ��Ʒ����Һ������ʯ��ˮ��ϴ��ƿ�� | Ʒ����Һ����ɫ������ʯ��ˮ����ǣ������к�Na2CO3���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(13�֣������������һ�ֳ����Ļ���ԭ�ϡ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3,���Ʊ���Ӧ����ʽΪ��
o
(1) �����ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����_______��
(2) �ø÷�����õ�Naj2O3^H2O�����г�����һ���������ʡ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
��������衿
����1 ��������ֻ��>fe2C03����
����2:������ֻ��Na2S����
����3: ____________________________
���������ϡ�
��NhS2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��
���ж���˼����
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4,��������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������_______ (�����������������������˵�����ɣ�____________________________
����Ʒ�������ʵ�顿
���ڼ���1,����±�ʵ�鷽���������ۣ�������ѡ)��
��ѡʵ���Լ�������KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
(3) ��֪����Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
�ĵ�ˮ���ж��ȡ���ζ������
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ����ʮУ�������ʽ������������ۺ��Ծ���ѧ���֣������棩 ���ͣ������
(13�֣������������һ�ֳ����Ļ���ԭ�ϡ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3,���Ʊ���Ӧ����ʽΪ��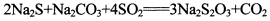 o
o
(1) �����ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����_______��
(2) �ø÷�����õ�Naj2O3^H2O�����г�����һ���������ʡ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
��������衿
����1 ��������ֻ��>fe2C03����
����2:������ֻ��Na2S����
����3: ____________________________
���������ϡ�
��NhS2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��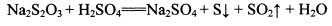
���ж���˼����
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4,��������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������_______ (�����������������������˵�����ɣ�____________________________
����Ʒ�������ʵ�顿
���ڼ���1,����±�ʵ�鷽���������ۣ�������ѡ)��
��ѡʵ���Լ���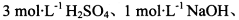 ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
(3) ��֪�� ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������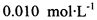 �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������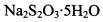 �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ����ʮУ�������ʽ������������ۺ��Ծ���ѧ���֣������棩 ���ͣ������
(13�֣������������һ�ֳ����Ļ���ԭ�ϡ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3,���Ʊ���Ӧ����ʽΪ��
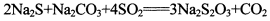 o
o
(1) �����ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����_______��
(2) �ø÷�����õ�Naj2O3^H2O�����г�����һ���������ʡ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
����1 ��������ֻ��>fe2C03����
����2:������ֻ��Na2S����
����3: ____________________________
��NhS2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��
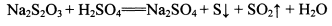
���ж���˼����
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4,��������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������_______ (�����������������������˵�����ɣ�____________________________
���ڼ���1,����±�ʵ�鷽���������ۣ�������ѡ)��
��ѡʵ���Լ���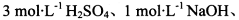 ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ

(3) ��֪��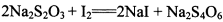 ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������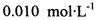 �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������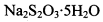 �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com