

A���÷�Ӧ���Ȼ�ѧ����ʽΪ��N2(g) + 3H2(g)  2NH3(g), ��H = -92kJ��mol-1 2NH3(g), ��H = -92kJ��mol-1 |
| B��b�����Ǽ������ʱ�������仯���� |
| C���������, �û�ѧ��Ӧ�Ļ�ܺͷ�Ӧ�ȶ��ı� |
| D�����¶����һ����������, ͨ��lmol N2��3molH2��Ӧ��ų�������ΪQ1kJ, ��ͨ��2molN2��6molH2��Ӧ��ų�������ΪQ2kJ ��184>Q2 >2Q1 |
 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH(l) �� 3/2O2(g) �� CO2(g) �� 2H2O(l)����H����725.8 kJ��mol��1 |
| B��2CH3OH(l) ��3O2(g) �� 2CO2(g) �� 4H2O(l)����H����725.8 kJ��mol��1 |
| C��CH3OH(l) �� 3/2O2(g) �� CO2(g) �� 2H2O(l)����H����725.8 kJ��mol��1 |
| D��2CH3OH(l) �� 3O2(g) �� 2 CO2(g) �� 4H2O(g)����H����1451.6 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
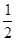 CH4(g)+O2(g)=
CH4(g)+O2(g)= 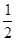 C02(g)+H2O(1)��?H =-445.15kJ��mol-1
C02(g)+H2O(1)��?H =-445.15kJ��mol-1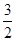 O2(g)=CO(g)+2H2O(1)�� ?H=-607.3kJ��mol-1
O2(g)=CO(g)+2H2O(1)�� ?H=-607.3kJ��mol-1| A��445.15 kJ��mol-1 | B��607��3 kJ��mol-1 | C��890��3 kJ��mol-1 | D��802��3 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2CO2(g)��Ӧ���̵������仯��ͼ��ʾ����֪1 mol CO (g)����Ϊ1 mol CO2 (g)�Ħ�H ����283 kJ/mol����ش��������⣺
2CO2(g)��Ӧ���̵������仯��ͼ��ʾ����֪1 mol CO (g)����Ϊ1 mol CO2 (g)�Ħ�H ����283 kJ/mol����ش��������⣺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2A ( l ) + B (l ) =" 2C" (g )��H1 | B��2A ( g ) + B ( g ) =" 2C" (g )��H2 |
| C��2A ( g ) + B ( g ) =" 2C" ( l )��H3 | D��2A ( l ) + B ( l ) =" 2C" ( l )��H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
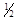 O2(g)==H2O(l)����H = ��285��8kJ��mol���ɴ˿�֪���ڵ���������45gҺ̬ˮ������____________kJ��������
O2(g)==H2O(l)����H = ��285��8kJ��mol���ɴ˿�֪���ڵ���������45gҺ̬ˮ������____________kJ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O(g) = H2(g)��1/2 O2(g)��H��+286kJ��mol-1 |
| B��2H2(g)+ O2(g)=2H2O(l)��H����572kJ��mol-1 |
| C��H2(g)+ 1/2O2(g)= H2O(g)��H����286kJ��mol-1 |
| D��H2(g)��1/2 O2(g)=H2O(l)��H��+286 kJ��mol-1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com