
| ��ʼŨ�ȣ�mol�� L-1�� | c�� NH3�� | ��c�� O2�� | c�� NO�� | c�� H20�� |
| �� | 1 | 2 | 0 | 0 |
| �� | 2 | 4 | 0 | 0 |
| �� | 0.5 | x | y | z |
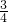 =6mL��
=6mL��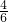 z=1��x+
z=1��x+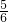 z=2�����x=1.375��y=0.5��z=0.75��
z=2�����x=1.375��y=0.5��z=0.75��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʼŨ�ȣ�mol�� L-1�� | c�� NH3�� | c�� O2�� | c�� NO�� | c�� H20�� |
| �� | 1 | 2 | 0 | 0 |
| �� | 2 | 4 | 0 | 0 |
| �� | 0.5 | x | y | z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡԭ��У������ѧ�ڵڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
�����仯���������ǵijԡ�����ס���С������ȶ��������е���ϵ��Ҳ�Ǹ��л�ѧѧϰ����Ҫ��һ���֡���ش��������⣺
I����1������һ֧15mL���Թܣ�����NO������ˮ���У����Թ��л���ͨ��һ�������������Թ���Һ���ȶ�ʱ��ʣ������3mL����ͨ���������������Ϊ ��
 ��2��һ�������£�ij�ܱ������з�����Ӧ��4NH3��g��+5O2��g��
4NO��g��+6H2O��g����
��2��һ�������£�ij�ܱ������з�����Ӧ��4NH3��g��+5O2��g��
4NO��g��+6H2O��g����
|
��ʼŨ�ȣ� mol/L�� |
C(NH3) |
C(O2) |
C(NO) |
C(H2O) |
|
�� |
1 |
2 |
0 |
0 |
|
�� |
4 |
8 |
0 |
0 |
|
�� |
0.2 |
x |
y |
z |
�ٺ��º����£�ƽ��ʱNH3��ת���ʼ� �ҡ������������=����������
�ں��º����£���Ҫʹ�����ƽ��ʱ�����Ũ����ͬ����x= ��y= ��z= ��
��3�����ݻ���ͬ���¶ȷֱ�ΪT1��T2�������ܱ������зֱ�������NO2��������Ӧ��2NO2��g�� N2O4��g����H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1< T2����a1_
a2��
N2O4��g����H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��a2����֪T1< T2����a1_
a2��
A������ B��С�� C������ D�����϶��п���
��4��2��24L����״����������200 mL l mol/L HNO3��Һ���պ�Ӧ����Һ�е�����Ũ�ȹ�ϵ�� ��
������������NF3����һ�����͵ĵ��Ӳ��ϣ����ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ������������HF�� NO�� HNO3������Ҫ��ش��������⣺
��1��д���÷�Ӧ�Ļ�ѧ����ʽ�� ����Ӧ�����У��������ͻ�ԭ�����ʵ���֮��Ϊ ��
��2������Ӧ������0��2mol HNO3��ת�Ƶĵ�����ĿΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ������
 4NO(g)+6H2O(g)
4NO(g)+6H2O(g) 
 N2O4(g) ��H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��
N2O4(g) ��H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������
 4NO(g)+6H2O(g)
4NO(g)+6H2O(g) 
 N2O4(g) ��H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1��
N2O4(g) ��H<0�����º����·�Ӧ��ͬʱ��ֱ�ⶨ��ϵ��NO2�İٷֺ����ֱ�Ϊa1���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com