

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
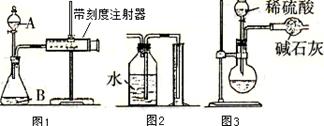
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
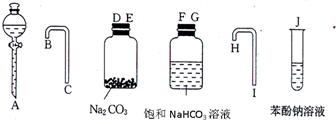
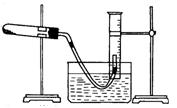
 ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ
����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װ ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
ͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý������õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺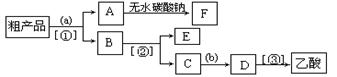
 ��
�� ��Һ�����뷽�����������뷽�����Ƿ�Һ
��Һ�����뷽�����������뷽�����Ƿ�Һ ��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�
��������Ҵ���������Ӧ�Ƿ�������Ӧ���еģ��䷴Ӧ�Ĺ������£�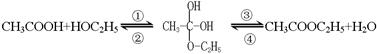
 ����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ��
����Ϊ��̽��������Ӧ�е���ˮ��ʽ��һ���ȡͬλ��ʾ�ٷ�������Ϊ��18Oͬλ�ؽ��б��ʱ�����б����ȷ���� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���˳����Բ��ò�ͬ��ұ�����������п����á��ȷֽⷨ��ұ���Ľ�����__________�� ��
���˳����Բ��ò�ͬ��ұ�����������п����á��ȷֽⷨ��ұ���Ľ�����__________�� �� ���ƺ���Υ�����˽������˳���Խ������ܽ������õļ��û�������ԭ��__________________________ ��
���ƺ���Υ�����˽������˳���Խ������ܽ������õļ��û�������ԭ��__________________________ �� �����ݲ�������Һ��ɫ��dz��һ��ʱ�����Һ
�����ݲ�������Һ��ɫ��dz��һ��ʱ�����Һ ���ǣ�������ط�Ӧ����ʽ���н���_________________________________��
���ǣ�������ط�Ӧ����ʽ���н���_________________________________�� ��ԭ�����ʵ����֤Cu2+��Fe3+������ǿ���Ľ��ۡ�
��ԭ�����ʵ����֤Cu2+��Fe3+������ǿ���Ľ��ۡ� ___________________________________________��
___________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
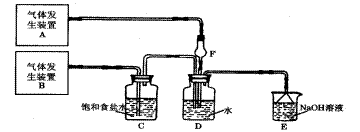
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����� | �Թܱ�� | ����ҩƷ | ʵ������ ����ɫʱ�䣩 |
| һ �����£� | 1 | 10ml 0.1mol/L�IJ��ᣨH2C2O4����Һ�������������ữ��0.1mol/L��KMnO4��Һ���� + ���������̹��� | 2s |
| 2 | 10ml 0.1mol/L�IJ��ᣨH2C2O4����Һ�������������ữ��0.1mol/L��KMnO4��Һ���� | 30s | |
| �� �����£� | 1 | 5ml 0.1mol/L�IJ��ᣨH2C2O4����Һ 5��0.1mol/L��KMnO4��Һ+10��ϡ���� | 90s |
| 2 | 5ml 0.1mol/L�IJ��ᣨH2C2O4����Һ 5��0.1mol/L��KMnO4��Һ | 100s | |
| �� ��65�� ��ˮԡ�� | 1 | 5ml 0.1mol/L�IJ��ᣨH2C2O4����Һ 5��0.1mol/L�� KMnO4��Һ +10��ϡ���� | 90s |
| 2 | 5ml 0.1mol/L�IJ��ᣨH2C2O4����Һ 5��0.1mol/L��KMnO4��Һ +1mlϡ���� | 100s | |
| 3 | 5ml 0.1mol/L�IJ��ᣨH2C2O4����Һ 5��0.1mol/L��KMnO4��Һ +2mlϡ���� | 120s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т٢� | B��ֻ�Т٢ܢ� | C��ֻ�Тڢܢ� | D���٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
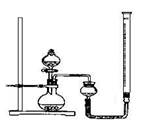
| ʵ����� | ˫��ˮ��� | ���� | �������� |
| a | 15mL | �� | |
| b | 15mL | 0.5g CuO | |
| c | 15mL | 0.5g MnO2 | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com