
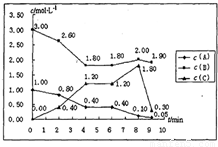
 ����v��C����
����v��C���� ������ƽ��ʱŨ�ȼ��㣻
������ƽ��ʱŨ�ȼ��㣻 =0.2mol/��L?min������2min����4min��v��C��=
=0.2mol/��L?min������2min����4min��v��C��= =0.4mol/��L?min����
=0.4mol/��L?min���� =
= =
= mol-1?L��
mol-1?L�� mol-1?L��
mol-1?L�� 2C��g����H=-XkJ/mol
2C��g����H=-XkJ/mol �����X=393.2��
�����X=393.2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
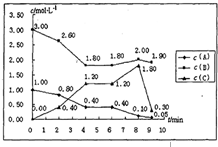 ��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��
��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��| 10 |
| 9 |
| 10 |
| 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ����ϰ���һ�и߶�������ڶ��νο��Ի�ѧ�Ծ����������� ���ͣ������
��4�֣�������ȼ��ʱ�ܷų��������ȣ�����Һ��ʯ��������Ҫ�ɷ֣���Ϊ��ԴӦ�������ǵ��ճ����������
��֪����2C3H8(g) +7O2(g) =" 6CO(g)" + 8H2O(l) ��H1= -2741.8kJ/mol
��2CO(g) + O2(g) = 2CO2(g) ��H2= -566kJ/mol
��1��д����ʾ��������ȼ���ȵ��Ȼ�ѧ����ʽ
��2������1mol C3H8�ڲ�������������ȼ�գ�����1mol CO��2mol CO2�Լ���̬ˮ�������еIJ���ͨ��һ���̶����Ϊ1L���ܱ������У���һ�������·������¿��淴Ӧ��
CO(g) + H2O(g)  CO2(g) + H2(g) ��H1=" +" 41.2kJ/mol
CO2(g) + H2(g) ��H1=" +" 41.2kJ/mol
5min����ϵ�ﵽƽ�⣬���ⶨ��H2Ϊ0.8mol�����(H2)= ���˹������յ�����Ϊ_____________��
��3�����ڷ�ӦCO(g) + H2O(g)  CO2(g) + H2(g) ��H1=" +" 41.2kJ/mol
CO2(g) + H2(g) ��H1=" +" 41.2kJ/mol
��һ�����ȵĹ̶��ݻ��������У��жϴ˷�Ӧ�ﵽƽ��ı�־�� ��
����ϵ��ѹǿ���ٷ����仯 �ڻ��������ܶȲ���
�ۻ�������ƽ����Է����������� �ܸ���ֵ����ʵ���Ũ�Ȳ��ٸı�
����ϵ���¶Ȳ��ٷ����仯 �ަ�(CO2)������(H2O)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com