
���б�������ȷ����(����)
A��NH4Cl��Һ��ˮ��������ԣ���NH4Cl���������
B��������Һ��ˮ����Լ��ԣ�ˮ������ӷ���ʽΪCO ��2H2OH2CO3��2OH��
��2H2OH2CO3��2OH��
C��������̼��������ԣ����뷽��ʽΪH2CO32H����CO
D������FeCl3��Һʱ���Ƚ�FeCl3���ڽ�Ũ�������У�Ȼ���ټ�ˮϡ�͵�����Ũ��
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��ĸ����õ绯ѧװ�õ�������ȷ����(����)


A��ͼ����ʾ����У�MnO2������
B��ͼ����ʾ��طŵ�����У�����Ũ�Ȳ�������
C��ͼ����ʾװ�ù��������У��������Һ��Cu2��Ũ��ʼ�ղ���
D��ͼ����ʾ����У�Ag2O�����������ڵ�ع��������б���ԭΪAg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һЩ������ȼ�������±���
| ������ | ȼ����/(kJ/mol) | ������ | ȼ����/(kJ/mol) |
| ���� | 891.0 | ������ | 2 878.0 |
| ���� | 1 560.8 | �춡�� | 2 869.6 |
| ���� | 2 221.5 | 2—������ | 3 531.3 |
���б�����ȷ����(����)
A���������ȼ���ȴ�Լ��3 540 kJ/mol����
B�����ȶ��ԣ������飼�춡��
C������ȼ�յ��Ȼ�ѧ����ʽΪ��2C2H6(g)��7O2(g)===4CO2(g)��6H2O(l)����H����1 560.8 kJ/mol
D����ͬ������������̼����������Խ��ȼ�շų�������Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
10��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
| �¶�(��) | 10 | 20 | 30 | ������к���ȴ��50�� |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
��ͬѧ��Ϊ������Һ��pH���ߵ�ԭ����HCO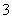 ��ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ__________________________��
��ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ__________________________��
��ͬѧ��Ϊ����ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�Na2CO3��ˮ��̶�__________(����ڡ���С�ڡ�)NaHCO3��
��ͬѧ��Ϊ�ס��ҵ��ж϶�����֡�����Ϊ��
(1)ֻҪ�ڼ�����е���Һ�м����������Լ�X����������������__________(��ס����ҡ�)�ж���ȷ���Լ�X��__________��
A��Ba(OH)2��Һ
B��BaCl2��Һ
C��NaOH��Һ
D�������ʯ��ˮ
(2)�����Ⱥ����Һ��ȴ��10�棬����Һ��pH__________(����ڡ��������ڡ����ڡ�)8.3����__________(��ס����ҡ�)�ж���ȷ��
(3)�������Ϸ���NaHCO3�ķֽ��¶�Ϊ150�棬������__________(��ס����ҡ�)�ж��Ǵ���ģ�������__________ _________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1 mol/L CH3COOH��Һ��ˮϡ�ͻ������CH3COONa���壬��������(����)
A����Һ��pH����
B��CH3COOH����̶ȱ��
C����Һ�ĵ�����������
D����Һ��c(OH��)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��p��qΪֱ����Դ������A�ɣ�2�۽�������X�Ƴɣ�B��C��DΪ���缫����ͨ��Դ������X������B����ͬʱC��D�������ݡ��Իش�
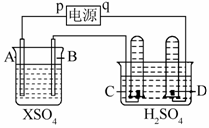
(1)pΪ__________����A��������__________(�Ӧ����)��Ӧ��
(2)CΪ__________�����Թ����ռ���__________��DΪ__________�����Թ����ռ���__________��
(3)C���ĵ缫����ʽ��_________________________________��
(4)�ڵ������У����C��D�����ϲ���������ʵ���������£�
| ʱ��(min) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| �������� �������(cm3) | 6 | 12 | 20 | 29 | 39 | 49 | 59 | 69 | 79 | 89 |
| �������� �������(cm3) | 2 | 4 | 7 | 11 | 16 | 21 | 26 | 31 | 36 | 41 |
��ϸ�����ϱ�����˵���õ�����ʵ�����ݿ��ܵ�ԭ����_________________________________________________________��
(5)����·��ͨ��0.004 mol����ʱ��B�缫�ϳ�������X������Ϊ0.128 g����˽�����Ħ������Ϊ______________________��
(6)����Ӧ����һ��ʱ���A��B�缫������Һ��pH__________(�������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������ϣ��ڹ����³�ַ�Ӧ�����ò����У���CH3Cl����CH2Cl2����CHCl3����CCl4����HCl��������ȷ���� (����)
A���٢� B���ڢ� C���٢ڢ۵Ļ���� D���٢ڢۢܢݵĻ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ţ�̷���ʱ�䳤�˻���ᣬ������Ϊţ���к��в������ǣ�����������������Ƿֽ��������ᡣ����������Ǵ���ţ���еõ����ɴ˶������ġ�����Ľṹ��ʽΪ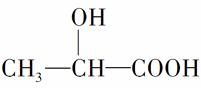 ������������⣺
������������⣺
(1)д����������й����ŵ����ƣ�______��______��
(2)д�����������������Ʒ�Ӧ�Ļ�ѧ����ʽ��
_____________________________________________________��
(3)д��������̼������Һ��Ӧ�Ļ�ѧ����ʽ��
_____________________________________________________��
(4)������Ũ���������£����������Ӧ������״�ṹ�����ʣ�д����������Ľṹ��ʽ��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������A����(ÿ������ֻ��һ����ԭ��)�����Լ�һ�ֻ���±�أ�����A��ˮ��Ӧ����ȫˮ�������������ԭ�����з�Ӧ�����������ˮ����A���ˮ��Һϡ�ͺ� �ֳɼ��ݣ��ֱ����һϵ�е��Լ�����������:
�ֳɼ��ݣ��ֱ����һϵ�е��Լ�����������:
�ټ����������������������ɫ������
�ڼ����Ȼ�����Һ����������;
����Һ���ữ�������������Һ����ɫ��ȥ���ټ������ᱵ��Һ��������ɫ������
(1)�ɴ��ж���ɸû������Ԫ���У����ܴ��ڵ�±����____________��A��ˮ��Ӧ�����ɵ���Һ�к��е����ӿ�����_________________________��
(2)Ҫȷ���û�����ķ���ʽ����ȡ11.90g A����ˮϡ����250.0mL��ȡ25.00mL��Һ���������ĸ��������Һ�����ᱵ��Һ��ʹ������ȫ��������ϴ�ӡ��������أ�Ϊ2.33g����ȷ��A�Ļ�ѧʽ��д��������̡�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com