
����Ŀ��H2C2O4Ϊ��Ԫ���ᡣ20������һ��c(H2C2O4)+c(HC2O4�C)+c(C2O42�C)=0.100 mol��L�C1��H2C2O4��NaOH�����Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ��һ����ȷ����
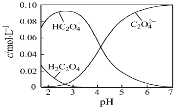
A��pH=2.5����Һ�У�c(H2C2O4)+c(C2O42�C)��c(HC2O4�C)
B��c(Na+)=0.100 mol��L�C1����Һ�У�c(H+)+c(H2C2O4)=c(OH�C)+c(C2O42�C)
C��c(HC2O4�C)=c(C2O42�C)����Һ�У�c(Na+)��0.100 mol��L�C1+c(HC2O4�C)
D��pH=7����Һ�У�c(Na+)<2c(C2O42�C)
���𰸡�B
��������
���������A������ͼ���֪��PH=2.5����Һ��c(H2C2O4)��c(C2O42-)Ũ��֮��С��c(HC2O4-)����c(H2C2O4)+c(C2O42-)��c(HC2O4-)����A����B��������Һ�е���غ�������غ������c(Na+)=0.100mol/L����Һ��ΪNaHC2O4��Һ����Һ�д��ڵ���غ�(H+)+c(Na+)=2c(C2O42-)+c(HC2O4-)+c(OH-)�������غ�c(Na+)=c(C2O42-)+c(HC2O4-)+c(H2C2O4)���������õ�c(H+)+c(H2C2O4)=c(OH-)+c(C2O42-)����B��ȷ��C��c(H2C2O4)+c(HC2O4-)+c(C2O42-)=0.100molL-1��c(HC2O4-)=c(C2O42-)������غ�(H+)+c(Na+)=2c(C2O42-)+c(HC2O4-)+c(OH-)��pHԼ4����ʱ������Ũ�ȴ������������õ���Һ��c(Na+)��0.100 molL-1+c(HC2O4-)����C����D��pH=7�����ݵ���غ�(H+)+c(Na+)=2c(C2O42-)+c(OH-)����������ˮ�⣬����c(Na+)��2c(C2O42-)����D��ȷ����ѡB��


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ڴ���������� ��
A����ˮ����� B������Ⱦ�Ŀ��� C������ D��Ư��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йص������Һ��˵����ȷ����
A. ��0.1mol![]() CH3COOH��Һ�м�������ˮ����Һ��
CH3COOH��Һ�м�������ˮ����Һ��![]() ��С
��С
B. ��CH3COONa��Һ��20��������30������Һ��![]() ����
����
C. �������м��백ˮ�����ԣ���Һ��![]()
D. ��AgCl��AgBr�ı�����Һ�м�������AgNO3����Һ��![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˾���ƺ�ݳ�����ͨ�����������������м��������������õĻ�ѧ��Ӧ���£�2CrO3(��ɫ)+3C2H5OH+3H2SO4��Cr2(SO4)3(��ɫ)+3CH3CHO+6H2O�����ڸ÷�Ӧ��������������ȷ����
A. ÿ1 mol CrO3����������Ӧ��ת��3 mol e��
B. C2H5OH�ǻ�ԭ��
C. CrO3�ڷ�Ӧ�б���������
D. C2H5OH�ڷ�Ӧ��ʧȥ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л�����һ�������¼ȿ������������ᣬ�ֿ��Ի�ԭ�ɴ�������ô������ɷ���ʽΪC4H8O2������������˵��������ǣ� ��
A�����л���������������л�ԭ��
B�����л����ܷ���������Ӧ
C�������л���ͷ���ʽC4H8O2������ɻ���ֻҪ������һ�����������������ߵ����ʵ���֮�ȣ���ȫȼ��ʱ�õ�������̼�����ض����
D�����л����������ͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������״̬�У�����֤�����淴ӦN2 + 3H2![]() 2NH3�Ѵ�ƽ��״̬��
2NH3�Ѵ�ƽ��״̬��
��һ��N��N�����ѵ�ͬʱ����3��H��H������
��һ��N��N�����ѵ�ͬʱ����6��N��H������
��������������ʱ����ϵѹǿ���ٸı�
�ܦ�(NH3)����(N2)�ͦ�(H2)�����ٸı�
�ݺ��º���ʱ���ܶȱ��ֲ���
�ަ���(H2)=0.03 mol��L-1��min-1������(NH3)=0.02 mol��L-1��min-1
A. ȫ�� B. �ڢۢܢ� C. �ۢܢݢ� D. �ڢۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������أ�K2FeO4����һ�����͡���Ч�����ˮ��������

��1����ҵ�ϵ�ʪ���Ʊ���������KClO��Fe��OH��3��KOH�������Ƶ�K2FeO4���÷�Ӧ�������뻹ԭ�����ʵ���֮��Ϊ________________��
��2��ʵ������ʳ�Ρ�����м�����ᡢKOH��Ϊԭ�ϣ�ͨ�����¹����Ʊ�K2FeO4��
�ٲ������ķ���Ϊ_________________������������ѹ���
�ڼ������X����ķ�����________________��
����������Һ�еõ�K2FeO4�������õ�ԭ����____________________��
��3���ⶨijK2FeO4��Ʒ������������ʵ�鲽�����£�
����1��ȷ����1.0g��Ʒ������100mL��Һ��
����2��ȷ��ȡ25.00mL K2FeO4��Һ���뵽��ƿ��
����3����ǿ������Һ�У��ù���CrO2����FeO42����Ӧ����Fe��OH��3��CrO42��
����4����ϡ���ᣬʹCrO42��ת��ΪCr2O72����CrO2��ת��ΪCr3+��Fe��OH��3ת��ΪFe3+
����5�������������������ָʾ������0.1000mol��L��1��NH4��2Fe��SO4��2����Һ�ζ����յ㣨��Һ���Ϻ�ɫ�����������ģ�NH4��2Fe��SO4��2��Һ���������3��ƽ��ʵ�飬ƽ�����ģ�NH4��2Fe��SO4��2��Һ�����30.00 mL��
��֪���ζ�ʱ�����ķ�ӦΪ��6Fe2+��Cr2O72����14H+=6Fe3+��2Cr3+��7H2O��
�ٲ���2��ȷ��ȡ25.00mL K2FeO4��Һ���뵽��ƿ�����õ�������______________��
��д������3�з�����Ӧ�����ӷ���ʽ__________________________��
�۲���5���ܷ�ָʾ��_________��ԭ����________________��
�ܸ�������ʵ�����ݣ��ⶨ����Ʒ��K2FeO4����������Ϊ__________��
��4������0.1mol��L-1��K2FeO4��������ҺpH������������ˮ��Һ�еĴ�����̬��ͼ��ʾ������˵����ȷ����__________ ������ĸ����

A��pH=2ʱ��c��H3FeO4+��+c��H2FeO4��+c��HFeO4����=0.1mol��L-1
B����pH��10��������Һ�м�����泥���HFeO4���ķֲ�����������
C����pH��1����Һ�м�HI��Һ��������Ӧ�����ӷ���ʽΪ��H2FeO4��H+��H3FeO4��
D����K2FeO4��������ˮ��ˮ��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ҫ�ɷ���Al2O3��SiO2��Fe2O3�ȡ���������������Al2O3������

����˵���д������
A. ��Һ�����Ҫ�ɷ���Na2SiO3��NaAlO2��NaOH
B. ��Һ��ĺ���������������HCO3-
C. ��ӦY�����ӷ���ʽ��2AlO2-��CO2��3H2O��2Al(OH)3����CO32-
D. ��Һ����ͨ�������X��Ŀ����ʹAlO2-��ֳ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����200��ʱ����a mol H2(g)��b mol I2(g)���뵽���ΪV L���ܱ������У�������Ӧ��I2(g)+H2(g)2HI(g)��
��1����Ӧ�տ�ʼʱ������c(H2)��______��c(I2)��______����c(HI)��________�����Ի�ѧ��Ӧ����________����________��С(Ϊ��)(�v������v����)��
��2�����ŷ�Ӧ�Ľ��У���Ӧ������и����Ũ�ȵı仯����Ϊc(H2)________��c(I2)________����c(HI)________���Ӷ���ѧ��Ӧ����v��________����v��________(���������С�����䡱)��
��3������Ӧ���е�v����v��________ʱ���˿��淴Ӧ�ʹﵽ������ȣ������������������ʱ��������и���ֵ����ʵ��������ʵ���Ũ�ȡ����������������������Ӧ���ת���ʺ�������IJ��ʼ���ϵ����ѹǿ(�����ֵķ�ѹ)����________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com