


 Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ʱ��������ƿ�̶��� |
| B������ʱ��������ƿ�̶��� |
| C�����ܽⲢ��ȴ����Һת��������ƿ���ֱ�ӽ��ж��ݲ��� |
| D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl2��HBr��HCl��H2 | B��Cl2��HCl��HBr��H2 |
| C��Cl2��HBr��H2��HCl | D��Cl2��H2��HCl��HBr |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
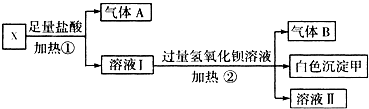
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| NaOH��Һ |
| ���� |
| ���� |
| ����NaOH��Һ |
| ���ˡ�ϴ�ӡ����ơ���ȴ |
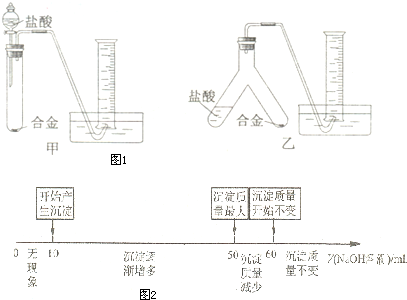
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

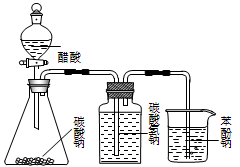
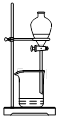

| A����װ�ã�����Ũ������Ҵ��������Ϊ��3��1�����Һ����ȡ��ϩ |
| B����װ�ã��Ƚϴ��ᡢ̼�ᡢ�������ߵ�����ǿ�� |
| C����װ�ã�����б��ӵı��м���NaOH��Һ����ȥ���еı��� |
| D����װ�ã�A��Ϊ�Ҵ������ᣬBΪ����̼������Һ����ȡ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
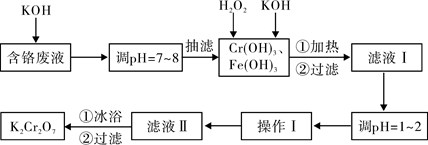
| ���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
| KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
| K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
| K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
| KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com