

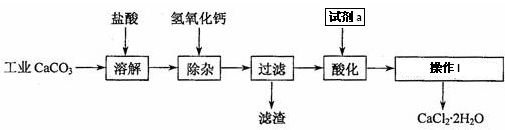
���� ���̷�����֪�������ࣨ��Ҫ�ɷ�ΪCr2O3������ΪFe2O3��Al2O3��SiO2��Ϊԭ�Ϸ�����ڷ�Ӧ���м���Ũ�����ȡ����������費��Ӧ�������˿ɵõ�����Ϊ�������裬��Һa�к���Fe3+��Al3+��Cr3+�ȣ�����̼������Һ����pH8.5��9.5���ɳ�ȥFe3+��Al3+���õ��ķ���ΪAl��OH��3��Fe��OH��3����CrO22-��Һ�м���������⣬�������ɵõ�CrO42-��Һ��Ȼ�����Pb��NO3��2��Һ���ɵõ�PbCrO4���������˺��Һ�к���Pb��NO3��2�����������ɵõ�����Ǧ��
��1��������������������������ĽӴ��������������߽�ȡ�ʣ�
��2��Ksp[Al��OH��3]=c��Al3+��•c3��OH-����ˮ��ƽ�ⳣ��Kh=$\frac{{c}^{3}��{H}^{+}��}{c��A{l}^{3+}��}$����Ksp[Al��OH��3]•Kh=��kw��3���ݴ˼��㣻
��3��������ͼ��֪������30%H2O2��Ŀ�����ڼ��������½�CrO2-����ΪCrO42-��ͬʱ����ˮ��
��4����������Ƿ���ȫ�ķ����ǣ����÷ֲ��ȡ�ϲ���Һ�������μ�Pb��NO3��2��Һ���������ɣ�˵��������ȫ��
��� �⣺���̷�����֪�������ࣨ��Ҫ�ɷ�ΪCr2O3������ΪFe2O3��Al2O3��SiO2��Ϊԭ�Ϸ�����ڷ�Ӧ���м���Ũ�����ȡ����������費��Ӧ�������˿ɵõ�����Ϊ�������裬��Һa�к���Fe3+��Al3+��Cr3+�ȣ�����̼������Һ����pH8.5��9.5���ɳ�ȥFe3+��Al3+���õ��ķ���ΪAl��OH��3��Fe��OH��3����CrO22-��Һ�м���������⣬�������ɵõ�CrO42-��Һ��Ȼ�����Pb��NO3��2��Һ���ɵõ�PbCrO4���������˺��Һ�к���Pb��NO3��2�����������ɵõ�����Ǧ��
��1��������������������������ĽӴ��������������߽�ȡ�ʣ��ʴ�Ϊ������Ӵ��������߽�ȡ�ʣ�
��2��Ksp[Al��OH��3]=c��Al3+��•c3��OH-��=1.3��10-33��ˮ��ƽ�ⳣ��Kh=$\frac{{c}^{3}��{H}^{+}��}{c��A{l}^{3+}��}$����Ksp[Al��OH��3]•Kh=��kw��3=��10-14��3=10-42������Kh=$\frac{1{0}^{-42}}{1.3��1{0}^{-33}}$=7.7��10-10���ʴ�Ϊ��7.7��10-10��
��3��������ͼ��֪������30%H2O2��Ŀ�����ڼ��������½�CrO2-����ΪCrO42-��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ3H2O2+2CrO2-+2OH-=2CrO42-+4H2O���ʴ�Ϊ��3H2O2+2CrO2-+2OH-=2CrO42-+4H2O��
��4����������Ƿ���ȫ�ķ����ǣ����÷ֲ��ȡ�ϲ���Һ�������μ�Pb��NO3��2��Һ���������ɣ�˵��������ȫ���ʴ�Ϊ�����÷ֲ��ȡ�ϲ���Һ�������μ�Pb��NO3��2��Һ���������ɣ�˵��������ȫ��
���� ���⿼�����ʵķ��롢�ᴿ���ۺ�Ӧ�ã�Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬����ע��ƽ�ⳣ���ļ��㡢���ӵļ��顢������ԭ��Ӧ��֪ʶ�㣬�ѶȲ������ӵļ����Ǹ߿����ȵ㣬ע�⣨4�����л�ѧ���Ե���ȷ���ã�Ϊ�״��㣮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʹƷ����Һ��ɫ | B�� | ����NaOH��Һ��Ӧ | ||
| C�� | ����H2O��Ӧ����H2SO4 | D�� | һ������������O2��Ӧ����SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| �е�/�� | -57.7 | 12.8 | - | 315 | - |
| �۵�/�� | -70.0 | -107.2 | - | - | - |
| �����¶�/�� | - | - | 180 | 300 | 162 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
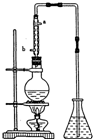
 ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
| �ܶȣ�g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� | |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ȩͨ��Ϊ40%���ҵ���ȩ��Һ�����õ���ȩ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�ļӺ��C2H4O��n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ����Һ���ױ�������Ϊ�ӱ��ʵ���ȩ��Һ����ȡ��ȩ���Եõ���Һ�������������·�Ӧԭ������C2H4O��n$\stackrel{{H}^{+}}{��}$ n��C2H4O��
������ȩͨ��Ϊ40%���ҵ���ȩ��Һ�����õ���ȩ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�ļӺ��C2H4O��n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ����Һ���ױ�������Ϊ�ӱ��ʵ���ȩ��Һ����ȡ��ȩ���Եõ���Һ�������������·�Ӧԭ������C2H4O��n$\stackrel{{H}^{+}}{��}$ n��C2H4O��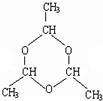 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO��/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com