
����Ŀ�������£�ijһԪ����HA�ĵ��볣��![]() �� ��
�� ��![]() Ũ��ԼΪ
Ũ��ԼΪ![]() ��Һ����μ���
��Һ����μ���![]() �ı�NaOH��Һ����pH�仯������ͼ��ʾ
�ı�NaOH��Һ����pH�仯������ͼ��ʾ![]() �����¶ȱ仯
�����¶ȱ仯![]() ����ش������й����⣺
����ش������й����⣺

��1��a��b��c��d�ĵ���ˮ�ĵ���̶�������________�㣬�ζ���������ѡ��__________��ָʾ�����ζ��յ���________������c������������c������������
��2���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע��______________________���ζ��յ������Ϊ_____________________________________________________��
��3�����ζ�����ʱ���ζ����е�Һ����ͼ��ʾ���������Ϊ___________mL��

��4���ζ������в��ֲ������£����и�����ʹ�������ƫ�ߵ���____________������ĸ��ţ���
A.�ζ�ǰ��ʽ�ζ���δ�ñ�NaOH��Һ��ϴ
B.������ˮϴ����ƿ������װ��HA��Һ����еζ�
C.�ζ������У���Һ���ֱ�ɫ������ֹͣ�ζ�
D.�ζ�����������Һ�棬��ȡNaOH��Һ���
��5�����ظ����εζ�ʵ����������±���ʾ������ζ�����HA��Һ�����ʵ���Ũ��Ϊ_____![]() ��
��
ʵ����� | NaOH��Һ��� | ����HA��Һ��� |
1 | 21.01 | 20.00 |
2 | 20.99 | 20.00 |
3 | 21.60 | 20.00 |
���𰸡�c ��̪ c������ ��ƿ�е���ɫ�仯 ���������һ�α�Һ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ 26.10 AD 0.1050
��������
(1)ˮ�ĵ��뷽��ʽΪH2O![]() H++OH-���ɸ���ƽ���ƶ�ԭ�����з�����ǿ��������յ�ʱ����ǿ�������Σ��ʼ��ԣ�Ӧѡ�ü��������±�ɫ��ָʾ����
H++OH-���ɸ���ƽ���ƶ�ԭ�����з�����ǿ��������յ�ʱ����ǿ�������Σ��ʼ��ԣ�Ӧѡ�ü��������±�ɫ��ָʾ����
(2)���ݵζ������淶���
(3)�ζ�������Ӧƽ�ӣ��ζ��ܶ���Ӧ����С�������λ��
(4)�ζ�������Ӧ���ݹ�ʽc(����)V(����)=c(��)V(��)������
(5)�����������Ʊ�Һ���Ӧ��ƽ��ֵ���м��㣬���㹫ʽΪ��c(����)V(����)=c(��)V(��)��
(1)ˮ�ĵ��뷽��ʽΪH2O ![]() H++OH-,��ˮ��Һ���������������ˮ�ĵ��룬���c��ˮ�ĵ���̶����ǿ��������յ�ʱ����ǿ�������Σ�ˮ��Һ�ʼ��ԣ��ζ��յ���c�����ϣ�ָʾ��Ӧѡ�÷�̪��
H++OH-,��ˮ��Һ���������������ˮ�ĵ��룬���c��ˮ�ĵ���̶����ǿ��������յ�ʱ����ǿ�������Σ�ˮ��Һ�ʼ��ԣ��ζ��յ���c�����ϣ�ָʾ��Ӧѡ�÷�̪��
(2)�ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע����ƿ�е���ɫ�仯;��̪��������������ɫ�����������º�ɫ����˵ζ��յ������Ϊ�����������һ�α�Һ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
(3)�ζ�����ÿһ��С�����0.10mL������ʱӦע��Ҫ����һλ��������Ϊ26.10��
(4)A.�ζ�ǰ��ʽ�ζ���δ�ñ�NaOH��Һ��ϴ,��Һ��Ũ��ƫ�ͣ�ʹ�ñ�Һ������ӣ����²ⶨ�����Ũ��ƫ�ߣ���A�������⣻
B.������ˮϴ����ƿ������װ��HA��Һ����еζ�����ʵ������Ӱ�죬��B���������⣻
C.�ζ������У���Һ���ֱ�ɫ������ֹͣ�ζ������ܵ��¼����������Ʊ�Һ���ƫС���������յζ����ƫ�ͣ���C���������⣻
D.�ζ�����������Һ���ȡNaOH��Һ��������¶�ȡ����������Һ���ƫ�����յζ����ƫ�ߣ���D�������⣻
��������������ӦѡAD;
(5)�������ݵ���Ч�ԣ���ȥ��3�����ݣ���ƽ�������������Ƶ����ΪV(��) = (21.01+20.99)mL/2=21.00mL�����ݹ�ʽc(����)V(����)=c(��)V(��)��֪��c(����)=0.021L��0.1000mol/L��0.020L=0.1050mol/L.


 �������ϵ�д�
�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������þ����mNa2SO4��nMgSO4��xH2O���׳����壬��ҵ���ð���þ���Ʊ���ʽ̼��þ[4MgCO3��Mg(OH)2��4H2O]����Ĺ������£���̼������Һ�м������þ��������50������0.5h���д�����5MgCO3��3H2O���ɣ�Ȼ�������¶ȵ�85�����2h�����ȽⷴӦ�����˵ü�ʽ̼��þ���塣
��1���ȽⷴӦ�ķ���ʽΪ________________��
��2����ʽ̼��þ��������ѧ��ȴ����ԭ����________________��
��3��Ϊ�ⶨ����þ������ɣ���������ʵ�飺
��ȡ����þ��3.340 g����ˮ���100.00 mL��ҺA��
��ȡ25.00 mL��ҺA�������������Ȼ�����Һ����BaSO4 1.165 g��
����ȡ25.00 mL��ҺA������pH��10����Ũ��Ϊ0.1000 mol��L��1��EDTA����Һ�ζ�Mg2+�����ӷ���ʽΪMg2+ + H2Y2����MgY2��+ 2H+�����ζ����յ㣬���ı���Һ25.00 mL��ͨ������ȷ������þ���Ļ�ѧʽ��д��������̣���___________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯���������������ѧ�о�����Ӧ�÷dz��㷺��
(1)��̬Feԭ�ӵļ۲���ӵĵ����Ų�ͼΪ_________________�����������ӵĵ�������״Ϊ___________��
(2)(NH4)2Fe(SO4)26H2O�׳�Ħ���Ρ�
��O��S��Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____________(��Ԫ�ط��ű�ʾ)��
��N��O��Ԫ�صĵĵ�һ�������ɴ�С��˳��Ϊ___________(��Ԫ�ط��ű�ʾ)��
��SO42-��Sԭ�ӵ��ӻ���ʽΪ___________��VSEPRģ������Ϊ___________________��
(3)Fe3+����ij�����ӷ�����������ɫ��Ӧ�����ڼ���Fe3+���������ӵĵ���ʽΪ_____���Ҽ��ͦм�����Ŀ֮��Ϊ______________����ռ乹��Ϊ__________��
(4)K3[Fe(CN)6]�����������ӵ���λ��Ϊ_____(�û�ѧ���ű�ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪ������ܵ�صĽṹʾ��ͼ��M��Na2O��Al2O3�Ƶã��õ�صĹ����¶�Ϊ320�����ң���ط�ӦΪ2Na��xS��Na2Sx������˵������ȷ����

A. ������ӦʽΪNa - e- = Na+
B. �ŵ�ʱ������32 g�����������ʣ�ת�Ƶĵ���Ϊ2 mol
C. M�������ǵ������������
D. ������ͬ�����ĸ����������ʣ������ص����۷ŵ�����Ǧ���ص�4.5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ij����������FeO��2Fe2O3��ĩ���Ƴ����ȼ����ֳ����ȷݡ�һ��ֱ�ӷ����������ռ���Һ�У���ַ�Ӧ��ų������ڱ�״���µ����Ϊ3.92 L����һ���ڸ�����ǡ�÷�Ӧ��ȫ����Ӧ��Ļ���������������ᷴӦ�ų��������ڱ�״���µ����Ϊ
A. 2.80 LB. 3.92 LC. 5.60 LD. 7.84 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ�
A. ��100 ����101 kPa�����£�Һ̬ˮ��������Ϊ40.69 kJ��mol-1����H2O(g)![]() H2O(l) ����H =" 40.69" kJ��mol-1
H2O(l) ����H =" 40.69" kJ��mol-1
B. ��֪MgCO3��Ksp =" 6.82" �� 10-6�������к��й���MgCO3����Һ�У�����c(Mg2+) = c(CO32-)����c(Mg2+) �� c(CO32-) =" 6.82" �� 10-6
C. ��֪��![]() ����Լ������Ӧ
����Լ������Ӧ ����HΪ��384 kJ��mol-1
����H��384 kJ��mol-1
D. �����£���0.10 mol��L-1��NH3��H2O��Һ�м�������NH4Cl���壬��ʹNH3��H2O�ĵ���Ƚ��ͣ���Һ��pH��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֶ�����Ԫ��X��Y��Z��M��N����ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ������˵��������ǣ� ��

A. �ǽ����ԣ�X>Z
B. ��̬�⻯����ȶ��ԣ�M<N
C. X��Y�γɵĻ�����ֻ���ܺ����Ӽ�
D. M����������Ӧ��ˮ������һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Щ��ѧ�����������21���͵���Դ��,����Ҫ��������Ϊ�뵼����ϵĹ���̫���ܷ�������о�����Ҫ�����á����й��ڹ��˵������ȷ���ǣ� ��
A. �ߴ��ȵĵ��ʹ豻�㷺�������������оƬ
B. ����ɶ������軹ԭ�Ƶ�
C. ����ʱ����ˮ�����������Ӧ,����������ᷴӦ
D. ��Ȼ���Ԫ�ص������ḻ,�����ڴ����ĵ��ʹ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2SO2(g)��O2(g)===2SO3(g)��Ӧ���̵������仯��ͼ��ʾ��
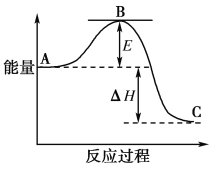
��֪1 mol SO2(g)����Ϊ1 mol SO3(g)����H����99 kJ��mol��1����ش��������⣺
(1)ͼ��A��C�ֱ��ʾ______________��________________��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ��________(����������������)Ӱ�죻
(2)�÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ�________��������________________________________________________________________________________��
(3)ͼ����H��________kJ��mol��1��
(4)��֪�������ȼ������H����296 kJ��mol��1��������S(s)����1 mol SO3(g)����H________(д���������)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com