
����Ŀ���������б��ش��й����⣺

��1���ϱ��У���һ��������С��Ԫ����__(��Ԫ�����ƣ���ͬ)���縺������Ԫ����__��
��2��ijԪ������������Ӧ��ˮ��������ԣ�д����Ԫ����cԪ���γɵĻ�������NaOH��Һ��Ӧ�����ӷ���ʽ__��
��3����e��kԪ�ص�ԭ���У�ԭ�Ӱ뾶��С����___(��Ԫ�ط���)����۵����Ų�ʽΪ__��δ�ɶԵ�����������__(��Ԫ�ط���)��������������Ӧˮ����Ļ�ѧʽΪ__��������δ�ɶԵ��ӵ�Ԫ����__(��Ԫ�ط���)��M���������չ������__(��Ԫ�ط���)��
���𰸡��� �� Al2O3+2OH-=2AlO2-+H2O Cl 3s23p5 P H3PO4 Si��S Al
��������
����Ԫ�������ڱ��е�λ�ÿ�֪��aΪCԪ�أ�bΪNԪ�أ�cΪOԪ�أ�dΪFԪ�أ�eΪNaԪ�أ�fΪMgԪ�أ�gΪA1Ԫ�أ�hΪSiԪ�أ�iΪPԪ�أ�jΪSԪ�أ�kΪC1Ԫ�أ�lΪArԪ�أ����Ԫ�������ɷ������
��1��ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�������A�塢��VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ�ͬһ����Ԫ���У����һ����������ԭ���������������С����һ��������С��Ԫ�����ƣ�ͬ����������ҵ縺������ͬ�������϶��µ縺�Խ��ͣ��縺������Ԫ���Ƿ����ʴ�Ϊ���ƣ�����
��2��ijԪ������������Ӧ��ˮ��������ԣ��û�����Ϊ�������������Ԫ����g����Ԫ����cԪ���γɵĻ�����Ϊ�������������������������NaOH��Һ��Ӧ����Ӧ�����ӷ���ʽΪ��![]() ��
��
��3��e��kԪ������ͬ����Ԫ�أ�ͬһ���ڣ��������ң�ԭ�Ӱ뾶��С��ԭ�Ӱ뾶��С����Cl��Ϊ17��Ԫ�أ���۵����Ų�ʽΪ3s23p5��δ�ɶԵ��������ļ۵����Ų�ʽΪ3s23p3����PԪ�أ�������������Ӧˮ����Ļ�ѧʽΪH3PO4��������δ�ɶԵ��ӵ�Ԫ�صļ۵����Ų�ʽΪ3s23p2��3s23p4��ΪSi��SԪ�أ�M���������չ���ļ۵����Ų�ʽΪ3s23p1����AlԪ�ء�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����t��ʱ��Ag2CrO4(�ٺ�ɫ)��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ����֪AgCl��Ksp��1.8��10��10������˵������ȷ����(����)
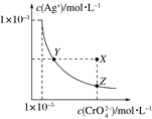
A.t��ʱ��Ag2CrO4��KspΪ1��10��8
B.����Ag2CrO4��Һ�м���K2CrO4����ʹ��Һ��Y���ΪX��
C.t��ʱ��Y���Z��ʱAg2CrO4��Ksp���
D.t��ʱ����0.01 mol��L��1AgNO3��Һ����20 mL 0.01 mol��L��1KCl��0.01 mol��L��1K2CrO4�Ļ����Һ�У�Cl���ȳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ�����
���� | �ṹ��ʽ | ||
��1�� | ___ | ��6�������� | ___ |
��2�� | ___ | ��7��������������ܶ�Ϊ46��ij������ | ___ |
��3�� | ___ | ��8������ | ___ |
��4�� | ___ | ��9������Ҫ�Ļ���ʯ��ˮƽ������ | ___ |
��5��HCHO | ___ | ��10��̼ԭ������3-6����������һ�ȴ���ֻ��һ�� | ___ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ����Ҫ�Ļ���ԭ�����Ա���Ϊ��Ҫ��ʼԭ�ϣ������з�Ӧ���Ƶ�����M�߷��ӻ�����N�������ֲ��P��Ӧ��������ȥ��

��1��B�Ĺ����ŵ�������_______________��
��2����֪C�ķ���ʽΪC5H12O��C�������Na��Ӧ��C�ĺ˴Ź���������3��壬��C�Ľṹ��ʽ ______________________��
��3����D����N�ķ�Ӧ������_________��B��C��Ӧ����M�ķ�Ӧ������_________��
��4��д��M��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________��
��5������F�Ľṹ�ǣ� �����������������F��ͬ���칹���� ______�֡�
�����������������F��ͬ���칹���� ______�֡�
���ܷ���������Ӧ ������NaHCO3��Һ��Ӧ ���������б������� �ṹ�����б�����ֻ������ȡ�������Һ˴Ź���������4��壬�����֮��Ϊ1:2:2:1��Ϊ___________(д�ṹ��ʽ)��
�ṹ�����б�����ֻ������ȡ�������Һ˴Ź���������4��壬�����֮��Ϊ1:2:2:1��Ϊ___________(д�ṹ��ʽ)��
��6����֪�� ��д���Ա��ӡ��Ҵ���ClCH2COOHΪԭ���Ʊ�
��д���Ա��ӡ��Ҵ���ClCH2COOHΪԭ���Ʊ�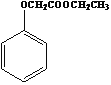 �ĺϳ�·������ͼ�����Լ����ã���___________
�ĺϳ�·������ͼ�����Լ����ã���___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽����������M������Һ�ķ�Ӧ��ijͬѧ����������ʵ�飬ʵ����̼�������ͼ��ʾ�����ʵ�������жϸ�ͬѧ�ó������н�������ȷ����

A. ����Һ�еμ������ʵ�������Dz�����ɫ����
B. �������������ֵ��ʵĻ����
C. ������������ϡ����������ȫ�ܽ�
D. ����Ľ���M������þ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȷ��ʾ���з�Ӧ�ķ���ʽ���ǣ� ��
A.![]() ��ˮ��Ӧ��
��ˮ��Ӧ��![]()
B.![]() ��Һ�м���
��Һ�м���![]() ��Һ��
��Һ��![]()
C.���ۺ�ˮ������Ӧ��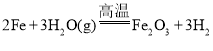
D.����![]() ����Һ�У�ͨ��
����Һ�У�ͨ��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() ��ʾ�����ӵ�������������������ȷ���ǣ� ��
��ʾ�����ӵ�������������������ȷ���ǣ� ��
A.���³�ѹ�£�![]() ���������е�ԭ��������
���������е�ԭ��������![]()
B.��״���£�![]() ������������Ϊ
������������Ϊ![]()
C.���³�ѹ�£�![]() ˮ����������Ϊ
ˮ����������Ϊ![]()
D.���³�ѹ�£�![]() ��
��![]() ����ﺬ�е���ԭ����Ϊ
����ﺬ�е���ԭ����Ϊ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��θҩ��ֹ���Ϊ̼��ƣ��ⶨÿƬ��̼��ƺ����ķ��������¼�����������ҩƬ�е������ɷֲ������ᷴӦ��Ҳ�����������Ʒ�Ӧ����ʵ�鲽�����£�
������![]() ϡ�����
ϡ�����![]() ��Һ��
��Һ��![]()
��ȡһ��ҩƬ��![]() ����������
����������![]() ����ˮ
����ˮ
�ۼ���![]() ϡ����
ϡ����
����![]() ��Һ�к������ᣬ��ȥ���Ϊ
��Һ�к������ᣬ��ȥ���Ϊ![]() ��
��
��ش��������⣺
��1���ⶨ�����з�����Ӧ�����ӷ���ʽ_________��
��2����������![]() ϡ�������ò�����������Ͳ���ձ���________��
ϡ�������ò�����������Ͳ���ձ���________��
��3�����ѡ�÷�̪��ָʾ�����ζ��ﵽ�յ������Ϊ____��
��4��ijͬѧ�Ĵβⶨ��![]() �������£�
�������£�
�ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| 13.40 | 11.90 | 12.10 | 12.00 |
�������λͬѧ��ʵ�����ݣ�����ҩƬ��̼��Ƶ���������Ϊ_____��
��5������ʵ������д������в�������ʹ����̼��Ƶ���������ƫ�ߵ���_________��
a ��û����ϴ�ļ�ʽ�ζ���װ![]() ��Һ���еζ�
��Һ���еζ�
b ��û����ϴ����ʽ�ζ�����ȡ![]() ϡ�����ܽ���Ʒ
ϡ�����ܽ���Ʒ
c ��![]() ��Һ�ζ�ʱ����ʼ����ƽ�ӣ��յ㸩��
��Һ�ζ�ʱ����ʼ����ƽ�ӣ��յ㸩��
d װ![]() ��Һ�ĵζ��ܣ��ζ�ǰ���������ݣ��ζ������������ݡ�
��Һ�ĵζ��ܣ��ζ�ǰ���������ݣ��ζ������������ݡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ�е��ܽ�����DO���Ķ����Ǻ���ˮ��ˮ�ʵ���Ҫָ�ꡣij��ѧС��ⶨij���������ĺ������������й������˽�ܽ����ⶨ����������������
������ȷ��������������ƣ�Na2S2O3����������һ�������cmol/L����Һ��
����ˮ��ƿȡ������ˮ��v1mL������������ע��1.0mLMnCl2��Һ��1.0mL����KI��Һ������ƿ����ƿ�ڲ������ݣ�����������Լ1Сʱ��
����ˮ��ƿ�м���1.0mL������Һ������ƿ������ˮ��ƿ������ȫ���ܽ⣬��ʱ��Һ��Ϊ��ɫ�� ������ˮ��ƿ����Һȫ��������ƿ�У�����������Ʊ���Һ�ζ���
V������Һ�ʵ���ɫ��1mL������Һ�������ζ����յ㲢��¼���ĵ������������Һ���Ϊv2��
��֪��I2 +2Na2S2O3 =2NaI+Na2S4O6
��1���ڵζ�������ʹ�õ������еζ��ܼС�����̨���ձ�����ƿ��________________________��
��2���ڲ�����У�ˮ���г�����MnMnO3���������ӷ���ʽΪ4Mn2++O2+8OH��![]() 2MnMnO3��+4H2O��
2MnMnO3��+4H2O��
��3��������з�����Ӧ�����ӷ���ʽΪ _______________________________________________________________��
��4���ζ�ʱ����Һ��__________ɫ��______________ɫ���Ұ��������ɫ���ٱ仯���ﵽ�ζ��յ㡣
��5����ˮ�е��ܽ���Ϊ_____________________________mg/L��
��6������ˮ�к��н϶�NO3��ʱ���ⶨ������ʵ��ֵ________(��ƫ�ߡ�ƫ�ͻ�)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com