
����12�֣�
��1����CO2Ϊ̼Դ��ȡ��̼�л���һֱ�ǻ�ѧ������о��ȵ㣬CO2������ȡ��̼���ķ�Ӧ���£�
��ӦI��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
��ӦII��2CO2(g)+6H2(g)=CH3CH2OH(g)+3H2O(g) ��H=��173.6kJ/mol
д����CH3OH(g)�ϳ�CH3CH2OH(g)�ķ�Ӧ���Ȼ�ѧ����ʽ_______________
��2��������أ�K2FeO4��������һ����Ҫ��������м�ǿ��������
�ٵ�ⷨ�ǹ�ҵ���Ʊ�K2FeO4��һ�ַ���������Ϊ�����������������Һ��Ȼ����������Һ�м���KOH�����ʱ����������Ӧ����FeO42-���õ缫��ӦʽΪ_________________
����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ�������������ϣ���缫��ӦʽΪFeO42-+3e-+4H2O=Fe(OH)3+5OH-����õ���ܷ�Ӧ�����ӷ���ʽΪ__________________
��3��amol FeS��bmol FeOͶ�뵽VL��C mol/L��������Һ�г�ַ�Ӧ����NO���壬���ó�����Һ�ɷֿɿ�����Fe(NO3)3��H2SO4�Ļ��Һ����Ӧ��δ����ԭ���������Ϊ_____
�٣�a+b����63g �ڣ�a+b����189g �ۣ�a+b��mol ��VC�� mol
mol
��1��2CH3OH(g)=CH3CH2OH(g)+H2O(g) ��H= -75.6kJ/mol
��2����Fe��6e- +8OH- = FeO42- + 4H2O
��3Zn + 2FeO42- + 8H2O = 2Fe(OH)3 + 3Zn(OH)2 + 4OH-
��3���ڢ�
��������
���������(1)��˹���ɣ����ܻ�ѧ��Ӧ��һ����ɻ�ּ�����ɣ��䷴Ӧ����ͬ������
2CH3OH(g)+2H2O(g) =2CO2(g)+6H2(g) ��H=��98.0kJ/mol ��
2CO2(g)+6H2(g)=CH3CH2OH(g)+3H2O(g) ��H=��173.6kJ/mol ��
��+�ڵ�2CH3OH(g)=CH3CH2OH(g)+H2O(g) ��H= -75.6kJ/mol
��2�������У�����������ӵĵ缫��������������������Ӧ���������ӵĵ缫����������������ԭ��Ӧ��
�ٴ�������Ϊ�������������������Һ������FeO42-���ʵ缫����ʽΪ
Fe��6e- +8OH- = FeO42- + 4H2O
����K2FeO4��Zn���Ե���У������õ��ӱ���ԭ����缫��ӦʽΪ
FeO42-��3e-��4H2O��Fe��OH��3��5OH-������Zn��������
��缫��ӦʽΪ��Zn��2e-��2OH-��Zn��OH��2����������ʧ�������غ��ϲ��ɵ��ܷ�Ӧʽ��2FeO42-��8H2O��3Zn��2Fe��OH��3��3Zn��OH��2��4OH-��
��3����Ӧ���������������ã�һ��������������ԭ��NO���塣���������������������������һ������������ϼ�û�仯��û�б���ԭ�����Ը��ݷ�Ӧǰ����������������֪����Ӧ����������������һ����֪����û����ԭ�������������Ӧǰ��a mol FeS��b mol FeO ��������ԭ���غ�֪����Ӧ��Fe(NO3)3�����ʵ����ǣ�a+b��mol����ô��������������ʵ�����3��a+b��mol������3��a+b��mol����û�б���ԭ����������
 ������ȷ��
������ȷ��
������Դӵ�ʧ���ӽǶȿ��ǡ�
FeS������Ϊ�����������ᣬ������1�ۣ�������8�ۣ�����a mol FeS��Ӧ��ʧȥ����9amol��FeO������Ϊ��������������1�ۣ�����b mol FeO ��Ӧ��ʧȥ����bmol��
ʧȥ�ĵ��ӱ�������õ����������ԭ��NO����Ԫ�ػ��ϼ۽���3�ۣ����ݵ�ʧ�����غ�֪��������ԭ����������ʵ����� mol����Ϊ����������n==CVmol������δ����ԭ����������ʵ�����
mol����Ϊ����������n==CVmol������δ����ԭ����������ʵ�����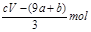 ���ܴ���ȷ��
���ܴ���ȷ��
���㣺��˹���ɡ�����ԭ����������������á�
���������⿼��ѧ���Ը�˹���ɵ��������գ��ڴ��������Ҫע����ŵ���д�Լ���Ч����
�ı������⣻����ԭ����Ҫ��ѧ��������صĵ缫����ʽ���ܵķ���ʽ������С�⣬��Ҫ
ѧ�����������ڷ�Ӧ�е�Ӧ�á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��2�֣���12�֣�.
��1������˵������ȷ���� ��
A.��100mL������ƿȷ��ȡ100mLҺ��
B.��Һʱ����Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ���
C.��������ƽ����NaCl����ʱ��NaCl���ڳ���ֽ�ϣ�����NaOH����ʱ��NaOH����С�ձ���
��2���ش��������⣨����ţ���
���������У���©��������ƿ��������ƿ����ƽ�ݷ�Һ©������Ͳ��ȼ�ճס�
���������ʷ������ �����и������ʷе㲻ͬ���������ʵ������� ��
��3�����ʵ����Լ�����ȥ���������������е��������ʣ�д���йصķ�Ӧ����ʽ��
��������������� ��
FeCl3�������FeCl2 ��
���� Na2CO3�������NaHCO3 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ���о�У������ѧ�ڵ�һ��������ѧ�Ծ� ���ͣ������
��ͼ��A��B��C��D��EΪ���ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��C+G��B+H���ų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�G�����Ǵ��������Ҫ�ɷ֣���I��һ�ֳ������������壬����E������Ӧ��2E+I��2F+D��F��EԪ�ص���������Ϊ60%���ش��������⣨ÿ��2�֣���12�֣�
��1����Ӧ�ٵĻ�ѧ����ʽ��
��2��������I�������ڵĻ�ѧ���� ��������ӡ����ԡ��Ǽ��ԡ���
��3����ȡ11.9gB��C��E�Ļ����ù�����NaOH��Һ�ܽ���ˡ�����ʣ���������Ϊ9.2g����ȡ��������B��C��E�Ļ������ϡ������ȫ�ܽ⣬���ռ������������6.72L����ʣ��Ļ��Һ�����������NaOH��Һʹ���еĽ���������ȫ�����������������Ϊ�� ��
A��27.2g B��7.8g C��2.7g D��19.4g
(4)C�������NaOH��Һ��Ӧ�����ӷ���ʽ�ǣ�
��5����G���ڹ�����ϡ�����У����������е�Fe3+�ķ����� �����������е�Fe2+�ķ�����
A���μ�KSCN��Һ����Һ��Ѫ��ɫ
B�������ۣ���Һ��dz��ɫ
C����������KMnO4��Һ��Ѹ����ɫ
D���μ�NaOH��Һ���а�ɫ������Ѹ�ٱ�ɻ���ɫ���ת��Ϊ�� ��ɫ
��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��������ˮһ�и���12���¿�������⻯ѧ�Ծ����������� ���ͣ������
����12�֣�
��1����CO2Ϊ̼Դ��ȡ��̼�л���һֱ�ǻ�ѧ������о��ȵ㣬CO2������ȡ��̼���ķ�Ӧ���£�
��ӦI��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
��ӦII��2CO2(g)+6H2(g)=CH3CH2OH(g)+3H2O(g) ��H=��173.6kJ/mol
д����CH3OH(g)�ϳ�CH3CH2OH(g)�ķ�Ӧ���Ȼ�ѧ����ʽ_______________
��2��������أ�K2FeO4��������һ����Ҫ��������м�ǿ��������
�ٵ�ⷨ�ǹ�ҵ���Ʊ�K2FeO4��һ�ַ���������Ϊ�����������������Һ��Ȼ����������Һ�м���KOH�����ʱ����������Ӧ����FeO42-���õ缫��ӦʽΪ_________________
����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ�������������ϣ���缫��ӦʽΪFeO42-+3e-+4H2O=Fe(OH)3+5OH-����õ���ܷ�Ӧ�����ӷ���ʽΪ__________________
��3��amol FeS��bmol FeOͶ�뵽VL��C mol/L��������Һ�г�ַ�Ӧ����NO���壬���ó�����Һ�ɷֿɿ�����Fe(NO3)3��H2SO4�Ļ��Һ����Ӧ��δ����ԭ���������Ϊ_____
�٣�a+b����63g �ڣ�a+b����189g �ۣ�a+b��mol ��VC�� mol
mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѵ���и�һ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ÿ��2�֣���12�֣�.
��1������˵������ȷ���� ��
A.��100mL������ƿȷ��ȡ100mLҺ��
B.��Һʱ����Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ���
C.��������ƽ����NaCl����ʱ��NaCl���ڳ���ֽ�ϣ�����NaOH����ʱ��NaOH����С�ձ���
��2���ش��������⣨����ţ���
���������У���©��������ƿ��������ƿ����ƽ�ݷ�Һ©������Ͳ��ȼ�ճס�
���������ʷ������ �����и������ʷе㲻ͬ���������ʵ������� ��
��3�����ʵ����Լ�����ȥ���������������е��������ʣ�д���йصķ�Ӧ����ʽ��
��������������� ��
FeCl3�������FeCl2 ��
���� Na2CO3�������NaHCO3 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ��һ5���¿���ѧ�Ծ��������棩 ���ͣ������
�����12�֣���1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��54 g D�ĵ��ʸ��������ᷴӦ������D3����67.2 L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��

��B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ�� ��
���õ���ʽ��ʾC��E�γ�E2C�Ĺ��̣� ��
��2��Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ���ͼ��Ԫ�����ڱ���һ���֡�
����Ӱ����Ԫ��N��Ԫ�����ڱ��е�λ��Ϊ ��
����һ�������£�S��H2��Ӧ��һ����(������Ϊ��Ӧ���еij̶�)�����жϣ�����ͬ������Se��H2��Ӧ���ȱ�S��H2��Ӧ�� ������ ��(ѡ���������С������ͬ��)
��Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ��Ӧ���ӷ���ʽ��__________________________________________��
������˵����ȷ����
A��C��N��O��F��ԭ�Ӱ뾶����ԭ���������������С
B��Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷������ڵĹ��ۼ�
D��HF��HCl��HBr��HI�����ȶ������μ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com