
����Ŀ��������ʵ����ʵ�����۽��ʹ�����ǣ� ��
ѡ�� | ʵ����ʵ | ���۽��� |
A | ���ԣ�H2SO4��H2SO3 | H2SO4���ǻ���ԭ�Ӹ�������H2SO3������ԭ��������Խ�ߣ�����Խǿ |
B | Be����������KOH��Һ | Be��Al�����ڱ����ǶԽ��߹�ϵ���������� |
C | Alԭ�ӵĵ�һ�����ܴ���Mgԭ�� | Mg�Ľ����Ա�Alǿ��������ʧȥ���� |
D | �е㣺���ǻ�����ȩ�����ǻ�����ȩ | ���ǻ�����ȩ���ڷ��Ӽ���������ǻ�����ȩ���ڷ�������������Ӽ���������Ƿе��Ӱ����ڷ�������������Ƿе��Ӱ�� |
A.AB.BC.CD.D
���𰸡�C
��������
A������ṹʽ��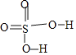 ��������ṹʽ��
��������ṹʽ��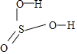 ��H2SO4���ǻ���ԭ�Ӹ�������H2SO3������ԭ��������Խ�ߣ�����Խǿ����A��ȷ��
��H2SO4���ǻ���ԭ�Ӹ�������H2SO3������ԭ��������Խ�ߣ�����Խǿ����A��ȷ��
B��Be��AlԪ�����ڱ�λ�ڶԽ���λ�ã����ݶԽ��߹������������ƣ��������ܹ���ǿ�Ӧ��֪Be�ܹ�������������Һ��Ӧ����B��ȷ��
C��ͬ���ڴ����ҵ�һ����������IIA��IIIA��������ΪMg�ļ۵����Ų�ʽ��3s2������ȫ��״̬�����ȶ�����Al��3s23p1�����Ե�һ�����ܣ�Mg��Al����C����
D�����γɷ��Ӽ���������ʷе�ϸߣ����ǻ�����ȩ�����γɷ�������������ǻ�����ȩ���γɷ��Ӽ�������������ǻ�����ȩ�ķе�ȶ��ǻ�����ȩ�ķе�ͣ���D��ȷ��
�ʴ�ΪC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ӷ���ʽBa2+��SO42-=BaSO4�����Ա�ʾ�� ��
�ٿ����Ա�����Һ���������������Һ֮��ķ�Ӧ
������������Һ���������������Һ֮��ķ�Ӧ
��ϡ����������Ա�����Һ֮��ķ�Ӧ
������������Һ��ϡ����֮��ķ�Ӧ
A.��B.�٢ڢ�C.�ڢ�D.�٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л���������ȷ����
A. 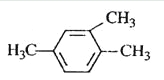 1,3,4-���ױ�
1,3,4-���ױ�
B. 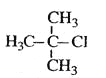 2-��-2-�ȱ���
2-��-2-�ȱ���
C. 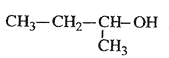 2-��-1-����
2-��-1-����
D. 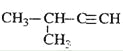 2-��-3-��Ȳ
2-��-3-��Ȳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.ԭ�Ӻ�������Ų�ʽΪ1s2��ԭ����ԭ�Ӻ�������Ų�ʽΪ1s22s2��ԭ�ӻ�ѧ��������
B.Fe3�������������Ų�ʽΪ3s23p63d5
C.��̬ͭԭ�Ӽ۵��ӵĹ����ʾʽ��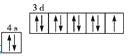
D.��̬̼ԭ�Ӽ۵��ӵĹ����ʾʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��״���£�4.48 L NH3����������Ϊ____________��___molCO2�к�����ԭ������1.806��1024��H2O���Ӻ��е���ԭ������ͬ
(2) 1.7g H2O2�к�����ԭ�ӵ����ʵ���Ϊ _________mol��������ĿΪ _____����֪ag H2O2�к�ԭ����Ϊb����٤������Ϊ _________(�ú�a��b�Ĵ���ʽ��ʾ)
(3)8.4 g N2��9.6 gij����Rx������ԭ�Ӹ�����ͬ���ҷ�����֮��Ϊ3:2����R�����ԭ��������_______��xֵΪ_______����д��Rԭ�ӵ�ԭ�ӽṹʾ��ͼ_________
(4)����µ�������O2��O3�����֮��Ϊ ______����ԭ�Ӹ���֮��Ϊ ______��
(5)��һ�ܱ������г���a molNO��b molO2���ɷ������·�Ӧ 2NO+O2=2NO2����ַ�Ӧ�������е�ԭ�Ӻ���ԭ�ӵĸ���֮��__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʯīϩ��Ŀǰ�Ƽ��о����ȵ㣬�ɿ�����ʯī�IJ�״�ṹһ��һ��İ����õ��ĵ���̼ԭ�ӣ��ṹ��ͼ��ʾ�������������뵽ʯīϩ�п�����һ�־���ͻ���Ե��²���ʯī�飬����˵������ȷ���ǣ� ��
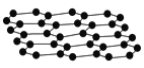
A.ʯīϩ�Ǹ߷��ӻ�����
B.12 g��ʯīϩ�к���3NA��C��C��
C.һ��������ʯīϩ����H2�����ӳɷ�Ӧ
D.���ݽṹʾ��ͼ��֪��ʯīϩ���ܵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������С�����������ZVI���ĵ绯ѧ��ʴ����������ϩ������ˮ�����Ĺ�����ͼ��ʾ��H+��O2��NO3���ȹ�����Ĵ��ڻ�Ӱ��ˮ����Ч�������嵥λʱ����ZVI�ͷŵ��ӵ����ʵ���Ϊnt������������Ч��ʴ�ĵ��ӵ����ʵ���Ϊne������˵��������ǣ� ��

A. ��Ӧ�٢ڢۢ�������������
B. ��λʱ���ڣ�������ϩ��ȥamolClʱne=amol
C. ���ĵ缫��ӦʽΪNO3��+10H++8e��=NH4++3H2O
D. ����λ���ˮ����С����ZVI��Ͷ��������ʹnt����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����廯�ѣ�TiBr4����������ҵ��ϩ���ۺϷ�Ӧ�Ĵ�������֪TiBr4������Ϊ�Ȼ�ɫ���壬�۵�Ϊ38.3�棬�е�Ϊ233.5�棬���г�����������ˮ�⡣ʵ�������÷�ӦTiO2+C+2Br2![]() TiBr4+CO2�Ʊ�TiBr4��װ����ͼ��ʾ���ش��������⣺
TiBr4+CO2�Ʊ�TiBr4��װ����ͼ��ʾ���ش��������⣺
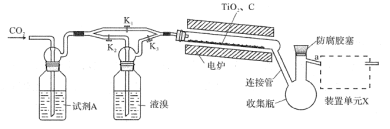
��1�����װ�������Բ�����ҩƷ����ǰӦ���еIJ�����__����Ŀ����__����ʱ����K1��K2��K3��״̬Ϊ__��һ��ʱ���¯�����ȷ�Ӧ�ܣ���ʱ����K1��K2��K3��״̬Ϊ____��
��2���Լ�AΪ__��װ�õ�ԪX��������__����Ӧ������������Դ��Ъ�������ӹܣ���Ŀ����___��
��3����Ӧ������Ӧ����ͨ��һ��ʱ��CO2����ҪĿ����___��
��4�������ӹ��жϲ��۷⣬���������ᴿ����ʱӦ��a�˵�������װΪ__���нӹܺͽ���ƿ���ڷ��������ϼ�װ��������___�����������ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH4����Cl-��Mg2����Ba2����CO32-��SO42-����ȡ���ݸ�100mL��Һ��������ʵ�飺
��һ�ݼ���AgNO3��Һ�г����������ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���0.08mol���塣�����ݼ�����BaCl2��Һ�õ��������12.54g������������ϴ�ӡ������������Ϊ4.66g��
��������ʵ�飬�ش��������⣺
��1���ɵ�һ�ݽ��е�ʵ���ƶϸû�����Ƿ�һ������Cl-��__��
��2���ɵڶ��ݽ��е�ʵ���֪�������Ӧ����__�������ʵ���Ũ��Ϊ__��
��3���ɵ����ݽ��е�ʵ���֪12.54g�����ijɷּ����ʵ�����__��
��4���ۺ�����ʵ�飬����Ϊ���½�����ȷ����__��
A.�û��Һ��һ������K����NH4��![]() ��CO32-��SO42-�����ܺ�Cl-����n(K��)��0.04mol
��CO32-��SO42-�����ܺ�Cl-����n(K��)��0.04mol
B.�û��Һ��һ������NH4����CO32-��SO42-�����ܺ�K����Cl-
C.�û��Һ��һ������NH4����CO32-��SO42-�����ܺ�Mg2����K����Cl-
D.�û��Һ��һ������NH4����SO42-�����ܺ�Mg2����K����Cl-
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com