
����Ŀ������Ҫ��ش��������⣺
�� ��Һ�������� �� ��
�� ![]() �۽��ʯ��C60 ��
�۽��ʯ��C60 ��![]() ��
��![]()
�������������У���Ϊͬ���칹�����_____����Ϊͬ�����������_____������ͬ�����ʵ���___________������ţ���
�����������������ʣ�a.NH4Cl b.ˮ�� c.Na2O2 d.�ɱ� e.C60������Ӧ��ĸ���:
��1�����ڷ��Ӿ������____________��
��2�����ڹ��ۻ��������______________��
��3���ۻ�ʱֻ��Ҫ�ƻ����ۼ�����_____________��
��4���Ⱥ������Ӽ��ֺ��й��ۼ�����__________________��
��5������b��e���,Ӳ�Ƚ�С����___________________��
���𰸡��� �� �� de bd b ac e
��������
��Һ������������ͬ�����ʵIJ�ͬ״̬���� ��
�� ![]() ������ʽC6H14����̼����ͬ�����Ի�Ϊͬ���칹�壬�۽��ʯ��C60����ͬ��Ԫ�صIJ�ͬ���ʣ���ͬ�������壬��
������ʽC6H14����̼����ͬ�����Ի�Ϊͬ���칹�壬�۽��ʯ��C60����ͬ��Ԫ�صIJ�ͬ���ʣ���ͬ�������壬��![]() ��
�� ![]() ��������ͬ����������ͬ��������ͬһԪ�صIJ�ͬ���ػ�Ϊͬλ�ء�����������������У���Ϊͬ���칹����Ǣڣ���Ϊͬ����������Ǣۣ�����ͬ�����ʵ��Ǣ١�
��������ͬ����������ͬ��������ͬһԪ�صIJ�ͬ���ػ�Ϊͬλ�ء�����������������У���Ϊͬ���칹����Ǣڣ���Ϊͬ����������Ǣۣ�����ͬ�����ʵ��Ǣ١�
����a.NH4Cl�������Ӿ��壬���й��ۼ������Ӽ����������ӻ�����ۻ�ʱ�ƻ����Ӽ���
b.ˮ������ԭ�Ӿ��壬ֻ���й��ۼ������ڹ��ۻ�����ۻ�ʱ�ƻ����ۼ���
c.Na2O2�������Ӿ��壬�������Ӽ����ۼ����������ӻ�����ۻ�ʱ�ƻ����Ӽ���
d.�����ڷ��Ӿ��壬������ֻ���й��ۼ������ڹ��ۻ�����ۻ�ʱ�ƻ����»����������
e.C60���ڷ��Ӿ��壬������ֻ���й��ۼ������ڵ��ʣ��ۻ�ʱ�ƻ����»�����
ԭ�Ӿ���Ӳ�ȴ������Ӿ��壬���Ӿ�����ڷ��Ӿ��壬ˮ����Ӳ�ȴ���C60��Ӳ�ȡ�
�����1�����ڷ��Ӿ������de��
��2�����ڹ��ۻ��������bd��
��3���ۻ�ʱֻ��Ҫ�ƻ����ۼ�����b��
��4���Ⱥ������Ӽ��ֺ��й��ۼ�����ac��
��5������b��e��ȣ�Ӳ�Ƚ�С����e��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������ʱ������˵����ȷ����
A. ʹ�ô���һ���ܼӿ췴Ӧ����
B. �÷�ĩ״п�����״п��ϡ���ᷴӦһ����ӿ췴Ӧ����
C. ��N2��3H2![]() 2NH3��Ӧ�У�����N2Ũ��һ������ʹH2ȫ��ת��ΪNH3
2NH3��Ӧ�У�����N2Ũ��һ������ʹH2ȫ��ת��ΪNH3
D. ����Ӧ����������Ũ�����ʱ�����淴Ӧһ�����ﵽ��ѧ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��ijҩ����м��壬��ṹ��ʽ��ͼ��ʾ�������й�X��˵����ȷ����
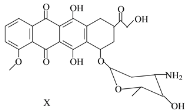
A. ÿ��X�����к���5������̼ԭ��
B. һ�������£�X�����Ҵ�����������Ӧ
C. һ�������£�X�ܷ�����ȥ��Ӧ
D. X���������ᷴӦ��������NaOH��Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���������÷����廯������������ʺϳ�ijҩ���ϳ�·�����£����ַ�Ӧ�Լ���������ʡ�ԣ���
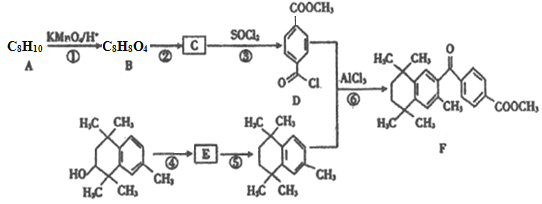
��֪��I. 
��. ![]()
�ش��������⣺
��1��B��������______________��
��2����Ӧ�������ķ�Ӧ����Ϊ______________��
��3����Ӧ�Ļ�ѧ����ʽΪ___________________________��
��4�������廯����X��C��ͬ���칹�壬ֻ��һ�ֹ�������1molX������ NaHCO3��Һ������Ӧ����2 molCO2����X�Ľṹ��_______�֡����к˴Ź���������ʾ��4��壬�ҷ����֮��Ϊ3:2:2:1�Ľṹ��ʽΪ______________��
��5��д�����Ҵ��ͼױ�Ϊԭ���Ʊ� ��·�ߣ��������Լ���ѡ��___________________��
��·�ߣ��������Լ���ѡ��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ú����ȼú�糧�ķ�������Ҫ�ɷ�ΪSiO2��Al2O3��Fe2O3��C�ȡ�ʵ����ģ�ҵ�ӷ�ú����ȡ����Al2O3������������ͼ��
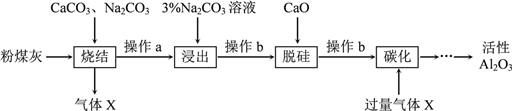
��֪�ս���̵IJ�����Ҫ����NaAlO2��Ca2SiO4��NaFeO2��Na2SiO3�ȡ�
��1��д���ս��������Ԫ��ת���Ļ�ѧ����ʽ_________________________��
��2������aΪ��ȴ����ĥ��������ĥ��Ŀ����________________________��
��3�����������У�NaFeO2����ȫˮ�⣬ˮ�ⷴӦ�����ӷ���ʽΪ_______________��
��4������b��������_________�����õIJ���������___________��__________���ձ���
��5����̼����ʱ���ɳ����������Ļ�ѧʽΪ________________��
��6������������ѭ��ʹ�õ�������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������״���;����㷺��Խ��Խ�����̼ҵĹ�ע����ҵ�ϼ״��ĺϳ�;�����ֶ���������ʵ������ģ��״��ϳɷ�Ӧ,��2 L�ܱ������ڣ�400 ��ʱ������ӦCO(g)+2H2(g) ![]() CH3OH(g)����ϵ��n(CO)��ʱ��ı仯�����
CH3OH(g)����ϵ��n(CO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 5 |
n(CO)(mol) | 0.020 | 0.011 | 0.008 | 0.007 | 0.007 |

��1��ͼ�б�ʾCH3OH �ı仯��������_______��
��2�����д�ʩ������߷�Ӧ���ʵ���_________(������Ӧ��ĸ���)��
a.�����¶� b.������� c.����ѹǿ d.��ʱ�����CH3OH
��3������������˵����Ӧ�ﵽƽ��״̬����__________(������Ӧ��ĸ���)��
a.CO��H2��Ũ�ȱ��ֲ��� b.v(H2)=2 v(CO)
c.CO�����ʵ����������ֲ��� d.�����������ܶȱ��ֲ���
e.ÿ����1molCH3OH��ͬʱ��2molH-H������
��4��CH3OH��O2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��ͼ��CH3OH��__________����A��B��ͨ�룬b���ĵ缫��Ӧʽ��__________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���һ�ֽϳ�ʹ�õĻ��ʣ����ڳ������ֽ⣮ij��ѧ��ȤС���̼淋ijɷִ������ʣ�ʱ��������̽����
������ʵ�飩������Һ�е�����������
ȡ������������Թ��У��������ᣬ�����ɵ�����ͨ�����ʯ��ˮ�У��а�ɫ�������ɣ�����ȡ����̼立����Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�������ɵ����壬ʯ����ֽ����ɫ��
��1������ʵ�������Ʋ�̼��������������ӿ�����_________��__________��
��2������ʵ������̼�������NaOH��Һ���ȷ�Ӧ�����ӷ���ʽ������________________��
���������飩�ⶨ̼���CԪ�غ�NԪ�������ȣ�����ȤС��ȷ��ȡag̼泥�����ʹ֮�ֽ⣬���Ѳ���ͨ���ʯ���У���ͼ1��ʾ��
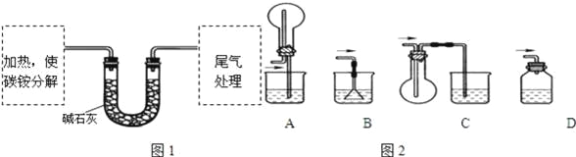
��3��̼粒���Ӧ����________�н��м��ȣ�
A���Թ� | B�������� | C����ƿ | D������ |
��4���Ӱ�ȫ�ĽǶȿ��ǣ�β��������װ�ÿ���ѡ����ͼ2�е�___________��
��5�������պ�û�й�����࣬����U�ι���ʵ��ǰ���������Ϊbg���ɴ˲��NԪ�ص�������_________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ܻ���NaOH��16.80gNaHCO3���壬��200�������³�ּ��ȣ��ų���Ӧ���������壬�õ����������Ϊbg��
(1)b����СֵΪ___________��
(2)��b=____________ʱ��������ˮ��������ࡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ����ף����ܺ�NO��CO2��NO2��NH3��N2�еļ��֣���100 mL�����徭����ͼ��ʾʵ��Ĵ���������õ�������Һ��������������ʣ�࣬����˵����ȷ���ǣ� ��

A. ������϶���NO2
B. ����Ũ���ᴦ����������NO��CO2�����Ϊ2:3
C. ���������ɿ���ΪNH3��NO��NO2
D. ����Na2O2������ʣ�������������80mL
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com