
�����ѣ�Ti������Ӳ�ȴ��۵�ߡ�����ʱ����ʴ�����㷺�������¿Ƽ����ϣ�����Ϊ��δ��������������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ��ұ��������ͬʱ��ø���Ʒ�Ĺ�ҵ�����������£��ش��������⣺
��1���������Ũ���ᷴӦ�IJ���֮һ��TiOSO4����Ӧ�����������ɡ�����Ʒ���׳ơ��̷����仯ѧʽ��________________��
��2���������������м���Feм��Ŀ���� �������ӷ���ʽ��ʾ�������鸱��Ʒ���Ƿ���ʵ�ʵ�鷽���� ��
��3�������������������õ��Ľ������л����������ʣ��ɼ��� �ܽ���ȥ��
��4����Һ���к���Fe2+��TiO2+������Mg2+�������ӡ������£����Ӧ���������Ksp���±���ʾ��
| �������� | Fe��OH)2 | TiO(OH)2 | Mg(OH)2 |
| Ksp | 8.0��10-16 | 1.0��10-29 | 1.8��10-11 |

�� FeSO4��7H2O �� 2Fe3��+Fe=3Fe2�� ȡ����Ʒ����������ˮ���μ�����KSCN��Һ���۲���Һ�Ƿ��ΪѪ��ɫ �� ϡ���� ��4����10 ��TiO2++2H2O=TiO(OH)2��+2H+ ��5���������������ڶ��������Χ�У����з�Ӧ�� ��6����TiO2 +4e-= Ti��2O2-
��������������� �̷��Ļ�ѧʽ��FeSO4��7H2O���� �������������м���Feм��Ŀ���Ƿ�ֹ��������������Ӧ�����ӷ���ʽ��2Fe3��+Fe=3Fe2�� �����鸱��Ʒ���Ƿ���ʵ�ʵ�鷽����ȡ����Ʒ����������ˮ���μ�����KSCN��Һ���۲���Һ�Ƿ��ΪѪ��ɫ������ΪѪ��ɫ֤�������ˣ�����δ���ʡ��� �����������������õ��Ľ������л�����������Mg���ɸ���þ�Ļ�Ա���ǿ���������ᷢ����Ӧ��ͨ�������������ܽ��ȥ���� Ksp=C(Mg2+)C2(OH-)=1.8��10-11;����ʼ������������=C(Mg2+)C2(OH-)> Ksp�� C2(OH-)=(1.8��10-11)��C(Mg2+)=(1.8c)��0.0018mol/L=1.��10-8,����C(OH-)=1��10-4.C(H+)=11��10-10.��PH="10." ����������Fe2+��TiO2+��Mg2+����Һ��ˮϡ�ͣ���������������ɫ�������÷�Ӧ�����ӷ���ʽ��TiO2++2H2O=TiO(OH)2��+2H+ ����5��Mg��ԭTiCl4�����б�����1070K���¶��½��У����ڸ�����Mg����������Ti���ױ����������ʣ��������ܲ���Ti�����Ի�Ӧ���Ƶķ�Ӧ�����Ǹ������������ڶ��������Χ�У����з�Ӧ�� ��6����TiO2 +4e-= Ti��2O2-��6��TiO2��������ʯī��������a�Ǹ�����b�������������ĵ缫��Ӧʽ��TiO2 +4e-= Ti��2O2-��
���㣺����Ti��ұ����������ȡ�������漰�ĸ����ʵĻ�ѧ���ʡ����ӵļ����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)ij����ÿ��Ҫ�յ�����1.6%����ú200 t,�ŷų���SO2��������Ⱦ����,������Ϊ��,����ЩSO2��������,��ô������ÿ��(��365 d��)������98%��Ũ������������;
(2)��Ҫ�����Ƽ��������,����Ӧ���Դ����������������,���Һ��������������Һ,��ƹ�������������Һ��Ũ�Ȼ���������(�������С�����䡱);
(3)��ҵ������ˮ�ࡢ����ʱ��Ҫ�õ���ԭ������������(����),����ѡԭ���Ʋ����Ļ�ѧ����ʽ���� ��;
| A������ | B����ʯ�� | C��ʯ��ʯ | D����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO������,��ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�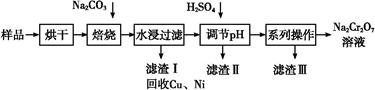
��֪:��Na2CrO4��Һ�к�������NaAlO2��Na2ZnO2������
(1)ˮ�������Һ������������(��ᡱ��������С�)��
(2)����������չ���������Na2CrO4�Ļ�ѧ����ʽ��
��������Cr(OH)3+��������Na2CO3+������������ ��������Na2CrO4+��������CO2+������������
��������Na2CrO4+��������CO2+������������
(3)���������Ҫ�ɷ���Zn(OH)2������������
(4)��ϵ�в�������Ϊ:��������H2SO4,��������,��ȴ�ᾧ,���ˡ���������H2SO4Ŀ������ ��
��֪:�ٳ�ȥ����II��,��Һ�д������·�Ӧ:
2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� �ܽ�� ��ѧʽ | 20 �� | 60 �� | 100 �� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ѺϽ��Ǻ��캽�չ�ҵ����Ҫ���ϡ�����������Ҫ�ɷ�Ϊ������������ѧʽΪFeTiO3���Ʊ�Ti�Ȳ�Ʒ��һ�ֹ�������ʾ��ͼ���£�
��֪���� TiO2����ˮ�⣬ֻ�ܴ�����ǿ������Һ�С� �ڳ����£����ܵ�����ܽ����pH��ϵͼ��
��25 ��ʱTiO(OH)2�ܶȻ�Ksp=1��10-29
�ش��������⣺
��1��д�����������ʱ����Ӧ�����ӷ���ʽ ��
��2������������ľ������������ ��
��3������TiO2����Һ���м���Na2CO3��ĩ�������� �� TiO2��ˮ������ӷ���ʽΪ ������ҺpH= ʱ��TiO(OH)2�ѳ�����ȫ��
��4��������м��Fe3+ת��ΪFe2+��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����÷���м(����������������������)������ʽ������[Fe(OH)SO4]�Ĺ����������£�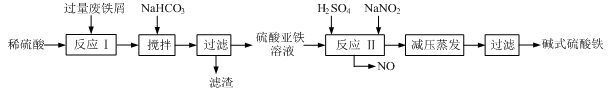
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4]����������Ͳ�Ѫ�������������������������£�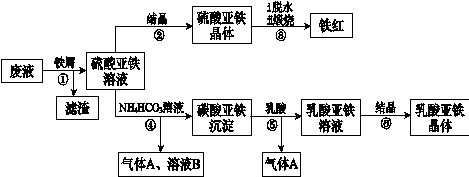
��֪��TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42������ش�
��1��������з�������������Һ�������IJ��������õIJ��������� ��
����ڵõ�������������IJ���Ϊ����Ũ���� ��
��1������ܵ����ӷ���ʽ�� ��
��1������ޱ������һ������նȣ�ԭ��������������ˮ�Լ� ��
��1�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ ��
��1����ƽ���ƶ���ԭ�����Ͳ�����м������ܵõ�����������ԭ�� ��
��1��Ϊ�ⶨ����������þ�����FeSO4��7H2O������������ȡ������Ʒa g������ϡ�������100��00 mL��Һ��ȡ��20��00 mL��Һ����KMnO4��Һ�ζ���������KMnO4����Ӧ����������0��1000 mol?L-1 KMnO4��Һ20��00 mL�����þ�����FeSO4��7H2O����������Ϊ����a��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�㶫ʡ���ŷḻ�ĺ�����Դ����ˮ��ȡʳ�κ�Br2�Ժ����±���������Ʊ�������MgCl2��MgO����±�к���Mg2����Cl��������������Na����Fe2����Fe3����CO(NH2)2�ȡ��Ʊ�������ͼ��ʾ��
(1)�����ijɷ���______(�ѧʽ)����Һ������������Ҫ�������� (д���ӷ���)��
(2)��NaClO��ȥ����CO(NH2)2ʱ������������⣬�����ܲ������ѭ�������ʣ���÷�Ӧ�Ļ�ѧ����ʽΪ________������NaClO������������______��
(3)ֱ�ӽ�MgCl2��6H2O��ǿ���ܵõ�MgO����Ӧ�Ļ�ѧ����ʽ��_____����MgCl2��6H2O�Ʊ���ˮMgCl2�����У�����Ҫ�Ļ�ѧ�Լ���________��
(4)��ˮ������������ճ�ʪ�����е�Br2������SO2���壬SO2����Br2�����ӷ���ʽ��_________________________________��SO2�������Դ�����Ṥҵ��β����ͬʱ��SO2β��Ҳ���ð�ˮ���գ���Ϊ�Ʊ����ʵ�ԭ�ϣ�SO2�����ð�ˮ���յõ��IJ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������Դ�����ԭ�������ʡ��������ŷŷ�����ȶ�����ɫ��ѧ�Ļ���Ҫ�����л�ʵ���У���������Ȼ�̼��Һ�������ˮ��Һ������ϡ�������Ũ�����ܽ⡢���������Թ��е���������ˮԡ���ȴ���ֱ���þƾ��Ƽ��ȣ��ܽӴ����������в��á��Ƚ�����������������Ԥ����ԭ��������ˮ���ݹ�ҵ�ϳɰ��з������õ������͵�����ѭ��ʹ�ã���ⷨұ���ƺ�þ��ѡ�����Ȼ��ƺ��Ȼ�þ��������Ӧ�Ľ��������������Ҫ���Ǵ���ɫ��ѧ�Ƕȿ��ǵ���( )
| A���٢� | B���ڢ� | C���ݢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com