
�����ʵ�����Ϊ0.1 mol��AlCl3��CuCl2��H2SO4����ˮ���Ƴ�100 mL�����Һ����ʯī���缫��⣬���ռ����缫�����������壬һ��ʱ����������ռ�������������ͬ�����µ������ͬ��������������ȷ����(����)
A����·�й�ת��0.9 mol����
B�������õ�����������O2���������ʵ���Ϊ0.35 mol
C����������������3.2 g
D����Ԫ�ؽ���Al(OH)3����ʽ����
A
���������������������ŵ磬��˳����Cu2����H������Cu2�����������õ���������ͭ���ʶ���������Cu2���ŵ����H�������������õ���������H2���ݳ����������������ŵ磬��˳����Cl����OH������Cl����������ʧȥ��������Cl2���ݳ�����Cl����ȫ�ŵ��OH������������ʧȥ��������O2���ų���������������ʵ����ʵ������ŵ�˳���������̿ɷֳ��ĸ��ν��У������ֱ�ΪCu��Cl2��H2��Cl2��H2��Cl2��Al(OH)3��H2��O2���ɵ�ʧ�����غ㡢�μӷ�Ӧ�ĸ����ӵ����ʵ����������õ�����������ͬ�����µ������ͬ����ͨ������ó���ȷ����A��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ ר��12��ѧʵ�������ϰ���������棩 ���ͣ�ѡ����
��ʵ�����н�������ʵ��̽�������е�ʵ����Ʒ�����õ�����ȷ����(����)��
ѡ��ʵ��̽������ʵ����Ʒ
Aͭ˿��������ȼ������ǯ��ȼ�ճס�������ͭ˿
B���ȷ�Ӧ����̨������������ͨ©�������ۡ�Fe2O3
Cʵ�����Ʊ������Թܡ�����ƿ���ƾ��ơ�NH4Cl��Ca(OH)2
D��KMnO4��������500 mL 0.1 mol��L��1 KMnO4��Һ ����ƿ���ձ�������������ʽ�ζ��ܡ�KMnO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ֳ�̷�ѡ��������ר�� �л��ƶ���ϰ���������棩 ���ͣ������
�ɽ���ۺ���P�ĺϳ�·�����£�

��֪��

(R����)
(1)A���������������________��
(2)����a�ĵ��뷽��ʽ��_______________________________________________��
(3)B��C�Ļ�ѧ����ʽ��_________________________________________________��
(4)������D�����ϵ�һ�ȴ�����2�֣�D�Ľṹ��ʽ��_______________________��
(5)E��F�з�Ӧ�������ķ�Ӧ���ͷֱ���________��
(6)F�Ľṹ��ʽ��_____________________________________________________��
(7)�ۺ���P�Ľṹ��ʽ��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ֳ�̷�ѡ��������ר�� ��ѧ����������ϰ���������棩 ���ͣ������
��ҵ����������Ϊԭ���Ʊ��������ѵĹ�����������ͼ��ʾ�����������Ҫ�ɷ�Ϊ��������(FeTiO3)������һ������Ԫ���ڷ绯�����л�ת��Ϊ��3�ۡ�
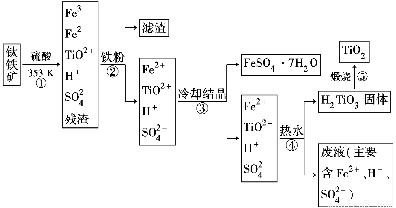
��֪��TiOSO4��ˮ��ˮ�⡣
(1)�������У������۽�Fe3��ת��ΪFe2�������ӷ���ʽΪ_______________________
(2)�������У�ʵ�ֻ����ķ������������ʵ�________(����ĸ���)��
a���ۡ��е���졡 b���ܽ��Բ��� �� c�������ԡ���ԭ�Բ���
(3)���������������У�����Ҫ���еIJ�����________(���������)��
(4)���ϻ�ѧ�����û�ѧƽ�����۽��Ͳ������н�TiO2��ת��ΪH2TiO3��ԭ����
____________________________________________________________��
(5)�������������еķ�Һ�����̿�(��Ҫ�ɷ�ΪMnO2)��Ӧ������������(MnSO4��������ˮ)���÷�Ӧ�����ӷ���ʽΪ__________________________________
(6)�о����֣���ʯī������������������������CaF2��CaO������ʣ�������ͼ��ʾװ�ÿɻ�ý����ƣ������Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
д�������ĵ缫��Ӧʽ��_________________________

���Ʊ�������ǰ��CaO���������䣬��ԭ����______________________________________(���ϻ�ѧ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ֳ��ѡ������ʱ����ר�� ����������ϰ���������棩 ���ͣ�ѡ����
��10 g Fe2O3��SiO2��Al2O3�Ļ�����У�����100 mL x mol/Lϡ���ᣬ���˺�����Һ�м���10 mol/L NaOH��Һ�����������������ͼ���NaOH��Һ����Ĺ�ϵ������ͼ��ʾ�����������������(����)

A��������ϡ���ᷴӦʱ��ϡ�������
B�������x��ֵ
C�������Al2O3����������
D�������Fe2O3����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ֳ��ѡ������ʱ����ר�� ����������ϰ���������棩 ���ͣ�ѡ����
��ҵ��ˮ�г�����һ������Cr2O72����CrO42�������ǻ����̬ϵͳ��ɺܴ�������л�ԭ�������dz��õ�һ�ִ����������������£�

\���е������д���ƽ�⣺2CrO42��(��ɫ)��2H�� Cr2O72��(��ɫ)��H2O�������й�˵����ȷ����(����)
Cr2O72��(��ɫ)��H2O�������й�˵����ȷ����(����)
A����������2v(Cr2O72��)��v(CrO42��)ʱ���ﵽ��ƽ��״̬
B����������ƽ�⣬��������ϡ�������Һ��ɫ���ɫ����������CrO42��������
C���������У���ԭ0.1 mol Cr2O72����Ҫ45.6 g FeSO4
D��������������a����ʹ��NaOH�ȼ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ����ר�⸴ϰ���ʵ���ɷ��༰�����仯��ϰ���������棩 ���ͣ�ʵ����
ijһ��θҩ�е������Ϊ̼���,��������������������IJⶨ����:
��������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ
��ȡһ��(ҩƬ������ͬ) 0.2 g�Ĵ�θҩƬ,ĥ������20.0 mL����ˮ
���Է�̪Ϊָʾ��,��0.1 mol��L-1��NaOH��Һ�ζ�,��ȥV mL��ζ��յ�
������25 mL 0.1 mol��L-1��HCl��Һ
(1)д��ʵ����̵IJ���(д���˳��)������������������������
(2)��ͼ��ʾ����������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ�϶�����Ҫ��������(�����)������,����������Һ����Ҫ�IJ���������������(����������)��

(3)����������ҺӦѡ�õ�����ƿ�����(����ĸ)������������
A.50 mL��50 mL
B.100 mL��100 mL
C.100 mL��150 mL
D.250 mL��250 mL
(4)д���йصĻ�ѧ��Ӧ����ʽ:����������������������������������
(5)ÿ��θҩ�к�̼��Ƶ�����Ϊ������ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ����ר�⸴ϰ ���ӷ�Ӧ��������ԭ��Ӧ��ϰ���������棩 ���ͣ�ѡ����
�������Ҫ�ɷ�Ϊ��ơ�̿�ۺ������,Ϊ��һЩ����Ч��������þ�ۡ����ۡ����ۡ���ۼ����Ρ�����˵���в���ȷ����(����)
A.����ը����ʹ��ˮ�����,���ø�ɳ
B.�����ȼ�Ų���������Ⱦ����,Ӧ��������
C.����ȼ�Ź������������������
D.����ը�����з�����ӦΪ2KNO3+3C+S K2S+N2��+3CO2��,ÿ����1 mol N2ת��10NA������(NA��ʾ�����ӵ�������ֵ)
K2S+N2��+3CO2��,ÿ����1 mol N2ת��10NA������(NA��ʾ�����ӵ�������ֵ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ����ר�⸴ϰ ���ʽṹ�����ʣ�ѡ��3����ϰ���������棩 ���ͣ������
A��B��C��D��E��FΪǰ������Ԫ����ԭ��������������,����A����3���ܼ�,��ÿ���ܼ������ĵ�������ͬ;C���������6���˶�״̬��ͬ�ĵ���;D�Ƕ�����Ԫ���е縺����С��Ԫ��;E������������ˮ����������ǿ;F�������ԭ�ӹ�����ڰ����״̬,�����ܲ���������ӡ�GԪ����DԪ��ͬ����,�����3�����ڡ�
(1)Ԫ��A��B��C�ĵ�һ��������С���������������(��Ԫ�ط��ű�ʾ)��
(2)E����ۺ�������E���ӻ���ʽΪ����������
(3)Fԭ�ӵ���Χ�����Ų�ʽΪ����������
(4)DE,GE���־���,���������Ӿ���,����λ����ͬ,��ԭ��������
(5)��֪DE����ľ�����ͼ��ʾ,����DE�����е�����E����ȥ��,����D����ȫ����ΪAԭ��,�������е�4����С�����������ĸ�����һ��Aԭ��,����4����С�������������ڡ�λ����С���������е�Aԭ���������4��Aԭ���Ե�������,�ɴ˱�ʾA��һ�־���ľ���(��֪A��A���ļ���Ϊa cm,NA��ʾ�����ӵ���������ֵ),��þ����к�������������Aԭ��,�þ�����ܶ�����������g��cm-3(��ʽ��ʾ)��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com