
��������ĵ���ƽ�ⳣ�����±���
���� | HCOOH | HCN | H2CO3 |
���볣����25�棩 | Ka=1��77��10?4 | Ka=4��9��10-10 | Ka1=4��3��10?7 Ka2=5��6��10?11 |
����ѡ�������ǣ� ��
A��CN�D+H2O+CO2=HCN+HCO3�D
B��2HCOOH+CO32�D=2HCOO�D+H2O+CO2��
C���к͵��������pH��HCOOH��HCN����NaOH����ǰ��С�ں���
D�����������Ũ�ȵ�HCOONa��NaCN��Һ��������������ǰ��С�ں���
D
��������
�����������ĵ���ƽ�ⳣ��HCOOH��H2CO3��HCN��HCO3-�����������ˮ��̶�CO32-��CN-��HCO3-��HCOO-��
��ĵ���ƽ�ⳣ��Խ���������Խǿ��ǿ���ܹ���ȡ���ᣬ���Զ��߷�Ӧ����HCN��HCO3-�����ӷ���ʽΪCN-+H2O+CO2�THCN+HCO3-��A��ȷ������ǿ��˳����HCOOH��H2CO3��HCN��HCO3-��ǿ���ܺ������η�Ӧ�������ᣬ���Է�Ӧ����ʽΪ2HCOOH+CO32-=2HCOO-+H2O+CO2����B��ȷ����pH���������HCOOH��HCN��n��HCN����n��HCOOH�����к�����Ҫ������ʵ�����������ʵ��������Ԫ�������ȣ������к͵��������pH��HCOOH��HCN����NaOH����ǰ��С�ں��ߣ�C��ȷ�����ݵ���غ㣬c��HCOO-��+c��OH-��=c��Na+��+c��H+����c��CN-��+c��OH-��=c��Na+��+c��H+����������������n��Na+ ��+n��H+����2������NaCN��ˮ��̶ȴ�NaCN��Һ�е�c��OH-����c��H+��С��c��Na+����ͬ�����Լ�����������������D����ѡD��
���㣺�������������ˮ��Һ�еĵ���ƽ�⡣


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�����Ȼ�ѧ����ʽ��
��1��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l) ��H1����870.3 kJ/mol��
��2��C(s)��O2(g)===CO2(g) ��H2����393.5 kJ/mol��
��3��H2(g)��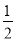 O2(g)===H2O(l) ��H3����285.8 kJ/mol��
O2(g)===H2O(l) ��H3����285.8 kJ/mol��
��Ӧ2C(s)��2H2(g)��O2(g)===CH3COOH(l)�Ħ�HΪ
A����488.3 kJ/mol B����244.15 kJ/mol
C����488.3 kJ/mol D����244.15 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�й�������ԭ��Ӧʵ�ʵ�˵������ȷ���ǣ� ��
A���Ƿ���Ԫ�صĵ���ת�� B���Ƿ���Ԫ�صĻ��ϼ۵ı仯
C���Ƿ�����Ԫ�صIJμ� D���Ƿ���ԭ�ӵ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ�߶���ѧ��ģ��һ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
����16�֣�ѡ��̪��ָʾ�������к͵ζ����ⶨ�ռ���Ʒ�Ĵ���(�������ʲ������ᷴӦ)��
�Ը���ʵ��ش�
��1��ȷ��ȡ4��8g�ռ���Ʒ��������������ҩ��,�ձ���,������ �� ��
��2�����ѳƺõ���Ʒ���250 mL����Һ����Ҫ�����������ձ�,���������� �� ��
��3����0��2000 mol / L������ζ������ռ���Һ���ζ�ʱ�ߵα�ҡ����ƿ������ע�� ��ֱ���ζ��յ㡣
��4���жϵ���ζ��յ��ʵ�������� ��
��5�������������ݼ��㣬�����ռ���Һ��Ũ��Ϊ ��(������������λ��Ч����)

��6�������������ݼ��㣬�ռ���Ʒ�Ĵ���Ϊ
��7�� �����ռ��������������ᷴӦ����Na2CO3����,���ռ���Ʒ���� (�ƫ�ߡ�, ��ƫ�͡�����Ӱ�족)
�������ָʾ���ɷ�̪���ɼ��ȣ����ռ���Ʒ���� (�ƫ�ߡ�, ��ƫ�͡�����Ӱ�족)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ�߶���ѧ��ģ��һ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪NaHSO3��Һ�����ԣ���Һ�д�������ƽ�⣺
HSO3�D+ H2O H2SO3 + OH�D �� HSO3�D
H2SO3 + OH�D �� HSO3�D  H+ + SO32�D ��
H+ + SO32�D ��
��0��1 mol �� L -1��NaHSO3��Һ�зֱ�����������ʣ������й�˵����ȷ����
A��������������Na��ƽ������ƣ�ƽ������ƣ���Һ��c(HSO3�D)����
B����������Na2SO3���壬��c(H+) + c(Na+) = c(HSO3�D) + c(OH�D) +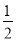 c(SO32�D)
c(SO32�D)
C����������NaOH��Һ��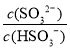 ��
��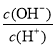 ��ֵ������
��ֵ������
D�����백ˮ�����ԣ���2c(Na+) = c(SO32�D)��c(H+) = c(OH�D)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ�߶���ѧ��ģ��һ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ñ�������Һ�ζ���������������Һʱ�����в����л�ʹ�ⶨ���ƫ�͵��ǣ� ��
A������ʽ�ζ��ܵ����յ�ʱ�����ӵζ��ܶ���
B������Һ������ƿ����10 ml����ˮ�ٵζ�
C����ʽ�ζ���������ˮ��ϴ��δ�ñ�������Һ��ϴ
D����ʽ�ζ���ע����Һʱ�����촦�������ݣ���ζ��յ�ʱ������ʧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ��ģ��һ���Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣�����NaCl��Na2SO4��NaNO3�Ļ����Һ��ѡ���ʵ����Լ�����ת��Ϊ��Ӧ�ij�������壬�Ӷ�ʵ��Cl����SO ��NO
��NO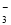 ������룬ʵ��������£�
������룬ʵ��������£�
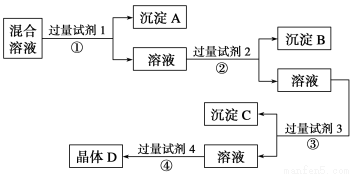
��ش��������⣺
��1��д������ʵ������������Լ������ƣ��Լ�1Ϊ_________���Լ�2Ϊ__________________���Լ�4Ϊ____________________��
��2����������Լ�3��Ŀ����__________________________________��
��3���ڼ����Լ�4��þ���D��ʵ������ܵ�����_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ��ģ��һ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͬ��ͬѹ�£�����������ܶ������ǣ� ��
A��O2 B��NO C��CO2 D��Cl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꼪��ʡ��һ��ѧ�����Ի�ѧ�Ծ��������棩 ���ͣ�������
������̼ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǣ��������ַ��������ǵ�ʯ��ˮ�����.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com