
 ���գ�
���գ� CH3COOH��Һϡ��100��������
CH3COOH��Һϡ��100�������� ��ҺpH a +2���>����<����
��ҺpH a +2���>����<���� ��
�� �����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ��
�����һЩ������֤��HA����������ʣ���ֻ�����д������������д�����岽�裬������Ŀ�ɲ�����Ҳ��������Ŀ�� ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ۢܢ� | B���ڢۢ� | C���ۢܢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������巴Ӧ�У������¶Ⱥ�����ѹǿ����ʹ��λ����ڵķ�Ӧ���л�������ͻ���Ӱٷ���ͬʱ���� |
| B��pH����7����Һһ�������� |
| C��c(H+)=c(OH-)����Һһ�������� |
| D����0.1mol/L�Ĵ�����Һ�У���������ˮƽ�������ƣ�����ƽ�ⳣ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Ksp��PbI2��>Ksp��AgCl�����Եó�PbI2��ˮ�е��ܽ�������AgCl�� |
| B�������£�ͬŨ�ȵ�Na2CO3��NaHCO3��Һ��ȣ�NaHCO3��Һ��pH�� |
| C�������ʵ���Ũ�ȵ�NH4Cl��Һ��NH4HSO4��Һ�����ߵ�c��NH4+���� |
| D��������pH=7�İ�ˮ������淋Ļ��Һ��c��NH4+����c��SO42-��֮��С��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Тں͢� | B��ֻ�Тں͢� | C��ֻ�Тٺ͢� | D��ֻ�Т� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

 Ũ����ô�����H
Ũ����ô�����H Ũ�ȵĹ�ϵ�� �� ��
Ũ�ȵĹ�ϵ�� �� ��| A��ǰ���Ǻ��ߵ�2�� | B��ǰ�߱Ⱥ��ߵ�2���� |
| C��ǰ�߱Ⱥ��ߵ�2��С | D�����ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A���к�10mL 1mol/L CH3COOH��Һ��Ҫ10ml 1mol/L NaOH��Һ |
| B����ʳ���Գ���ˮƿ�ڵ�ˮ�� |
| C��pH=2�Ĵ�����Һϡ��1000����pHС�ڣ� |
| D����ŨH2SO4�ʹ����ƹ��干�ȿ��Ƶô��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
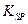 =2.6��10-39��Mg(OH)2���ܶȻ�����
=2.6��10-39��Mg(OH)2���ܶȻ�����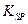 =5.6��
=5.6��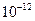 ��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��
��ȡ����Ũ�Ⱦ�Ϊ0.1mol/L��MgCl2��FeCl3���Һ����HCl��������һ������MgCO3�ﵽ������Һƽ�⣬���pH=4.00������¶��²�������Һ�е�c(Fe3+)=______________��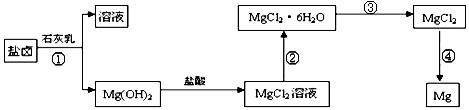
 .���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl��������
.���̢۵�ת����Ҫ��HCl�����м��ȣ�HCl�������� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��10 mL 0.10 mol��L-1��ˮ��10 mL 0.10 mol��L-1������ c��Cl-��> c��NH4+��> c��OH -�� > c��H+�� |
| B��10 mL 0.10 mol��L-1 NH4Cl��Һ��5 mL 0.20 mol��L-1 NaOH��Һ��� c��Na+�� = c��Cl-]��> c��OH -��> c��H+�� |
| C��10 mL 0.10 mol��L-1 CH3COOH��Һ��5 mL 0.20 mol��L-1 NaOH��Һ��� c��Na+��= c��CH3COO-�� > c��OH -�� > c��H+�� |
| D��10 mL 0.50 mol��L-1 CH3COONa��Һ��6 mL 1.0 mol��L-1������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com