
����Ŀ����ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2,װ����ͼ��ʾ(�г�װ��ʡ��)��
��֪:��Mg��Br2���ҷ�Ӧ���ų���������; MgBr2 ����ǿ��ˮ��;
��MgBr2 + 3C2H5OC2H5![]() MgBr2��3C2H5OC2H5��
MgBr2��3C2H5OC2H5��

ʵ����Ҫ��������:
����1:������ƿ��װ��10 gþм(þ����ĥ���������)��
150 mL��ˮ����;����B�м���15 mLҺ�壬����װ��;��
����2:��ֹˮ�У�����ͨ�˸���ĵ�����ֱ������ȫ����������ƿ��;
����3:��Ӧ��Ϻ�ָ������£����˳�ȥþ����Һת������һ�������ƿ�У���ȴ��0��C������.���壬���˵������Ѻ��廯þ�ֲ�Ʒ;
����4:�ñ�ϴ�Ӵֲ�Ʒ����ѹ���ˣ��������Ѻ��廯þ�����������1609��C�ֽ����ˮMgBr2��
�ش���������:
��1��MgBr2 ���γɹ��̿��õ���ʽ��ʾΪ_____________
��2������A��������_______;����B��������___________.ʵ��ǰ����A��B��������ƿ�ڱھ��豣�ָ��ԭ����__________________
��3��ʵ����,______________(������������������)�ø������������ﵪ��,������___________��
��4����ȥ����ˮԡ��������ƿ������MgBr2��ͬʱ�����ܻ�������������X, 1 mol X��50 mol e-,�仯ѧʽΪ______________________________________________��
��5������4���ü�ѹ����(����������ѹǿ��ʹ��Һ���ٷ���)������װ�ÿ�������ѹ���˵���________________________(�����)��

��6��Ϊ�ⶨ��Ʒ�Ĵ���(�ٶ����ʲ����뷴Ӧ)������EDTA (��дΪY4-����ɫ)����Һ�ζ��������TΪָʾ��(pH=6.3~11.6ʱ����ɫ,pH>11.6ʱ�Գ�ɫ)����֪: Mg2+�����T�γɵ������(Mg2+_���T)�ʾƺ�ɫ��Mg2+��Y4-�γɵ�MgY2-Ϊ��ɫ;��pHԼΪ9�Ļ�����Һ�еζ�����Ӧ�����ӷ���ʽ�ɼ�ʾΪ: Mg2+ + Y4-=MgY2-, Mg2+-��� T+Y4- =MgY2- +���T��
���жϵζ��յ������Ϊ__________________.
�ڲⶨǰ���ȳ�ȡ0.2500g��ˮMgBr2��Ʒ���ܽ����2�����T��Һ��ָʾ������0. 0500 mol��L-1 EDTA����Һ�ζ����յ㣬�ظ����εζ���ƽ������EDTA����Һ26. 60 mL,������ˮMgBr2��Ʒ�Ĵ�����___________________.
���𰸡�![]() �����������Ѻ������� ��ƿ MgBr2����ǿ��ˮ�ԣ���Ҫ����װ�ñ��ָ��� ���� þм��������Ӧ����MgO����Ĥ�������������谭Mg��Br2��Ӧ Mg3N2 bc ���μ����һ��EDTA����Һʱ����Һ�ɾƺ�ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ֲ��� 97.89%
�����������Ѻ������� ��ƿ MgBr2����ǿ��ˮ�ԣ���Ҫ����װ�ñ��ָ��� ���� þм��������Ӧ����MgO����Ĥ�������������谭Mg��Br2��Ӧ Mg3N2 bc ���μ����һ��EDTA����Һʱ����Һ�ɾƺ�ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ֲ��� 97.89%
��������
��ʵ��Ҫ�Ʊ���ˮ�廯þ��������Ŀ��Ϣ��֪þ�������ҷ�Ӧ�����ų��������ȣ�������װ��Ϊ�ܱ���ϵ������ͨ����岻��̫�죬�Է����ҷ�Ӧ������ը�����Ը���ĵ���Ҫ����ͨ�룬��Bװ���е���Ҫ�����̳����廯þ����ǿ��ˮ�ԣ���������װ��Ҫ������ˮ��������Ҫװ�ø���ܷ�ֹ�����е�ˮ�������룻��Ӧ�л�ų������ȣ�Ϊ�˼�����ԭ�ϻӷ���ɵ���ģ���Ҫ��������������������ҩƷ��
(1)�廯þ�γɹ�����þԭ��ʧȥ�������ӣ�������ԭ�ӷֱ�õ�һ�������γ������ӣ�Ȼ��������������þ���ӽ�������廯þ���õ���ʽ��ʾΪ��![]() ��
��
(2)װ��AΪ���������ܣ�����������ӷ������������ܿ��������������Ѻ�������������B�Ľṹ��֪��Ϊ��ƿ��������Ŀ��Ϣ��֪MgBr2����ǿ��ˮ�ԣ���Ҫ����װ�ñ��ָ��
(3)��ʵ��Ҫ��þм��Һ�巴Ӧ�����廯þ������װ���в���������þ��Ӧ�����壬�����������ø���Ŀ����������ĵ�����þм��������Ӧ����MgO����Ĥ�������������谭Mg��Br2��Ӧ��
(4)����Ԫ���غ��֪������ӦΪMgԪ�غ�NԪ���γɵ����ʣ��ٽ��1mol�����ʺ���50mol���ӿ�֪������ӦΪMg3N2��
(5)��ѹ���˹�������Ҫ����ƿ���γɸ�ѹ�����Կ���������ѹ���˵�װ��Ϊbc��
(6)�ٸ��ݵζ�ԭ��������ָʾ����Mg2+�����T�γɵ������(Mg2+-���T)����ʱ��ҺΪ�ʾƺ�ɫ���ζ������з�����ӦMg2+-��� T+Y4- =MgY2- +���T���ζ��յ�����pH=9����Һ��ʾ����T����ɫ�����յ�����Ϊ�����μ����һ��EDTA����Һʱ����Һ�ɾƺ�ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ֲ��䣻
�����ݷ���ʽMg2++Y4-�TMgY2-�������廯þ�����ʵ���=0.0500mol/L��0.02660L=0.00133mol�����廯þ������Ϊ0.00133mol��184g/mol=0.24472g���廯þ�IJ�Ʒ�Ĵ���=![]() ��100%=97.89%��
��100%=97.89%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)���ʹ�õ��ƽ���ȼ����N��H����Ԫ����ɣ���ԭ�Ӹ���N��H=1��2����ˮ��Һ�Լ��ԣ����������Nԭ�ӵ��ӻ���ʽΪ______________________��
(2)Ц��(N2O)������������������������ʳ�ᵼ������������ҡ�Ԥ��N2O�ĽṹʽΪ________________________��
(3)Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��1������ʱ���ų�������������һ��������(E)����1���������ٻ��һ�����ӵ������仯�����ڶ��������ܣ�����Ԫ�ػ����ӵĵ��������������±���ʾ��
Ԫ�� | C1 | Br | I | O | O- |
�������ܣ�kJ��mol�� | 349 | 343 | 295 | 141 | ��780 |
����˵����ȷ����___________��
A����������Խ��˵��Խ�ѵõ�����
B��һ����̬����̬��ԭ�ӵõ�һ�����ӳ�ΪO2-ʱ�ų�141kJ������
C����Ԫ�صĵڶ����������ǣ�780kJ��mol
D����̬����̬��ԭ�ӵõ��������ӳ�ΪO2-��Ҫ��������
(4)�ڵ�����������м������ʯ(����A������)��������Al2O3�۵�����á�����ʯ������ԭ��Ϊ��2Al(OH)3+12HF+3Na2CO3=2A+3CO2��+9H2O�������������������գ�
�ٱ���ʯ�Ļ�ѧʽΪ____________________________��
�ڱ���ʯ�����������ɣ�����ʯ�ľ����ṹ��ͼ����ʾ����λ�ڴ�������Ķ�������ģ���λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ�������������___________(��������)��
�۱���ʯ��Һ�в����ڵ�������������________________(��ѡ����ĸ)��
A ���Ӽ� B ���ۼ� C ��λ�� D ������ E ���»��� F ���
��Al���ʵľ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��

����֪A1��ԭ�Ӱ뾶Ϊd cm��NA���������ӵ�������Al�����ԭ������ΪM������Alԭ�ӵ���λ��Ϊ________��Al������ܶ�Ϊ__________g��cm-3(����ĸ��ʾ)��
(5)�����Fe(CO)5���۵㣭20�棬�е�103�棬�������Ʊ�������Fe(CO)5�Ľṹ��ͼ��ʾ��

��Fe(CO)5������������__________���塣
�ڹ���Fe(CO)5������˵����ȷ����_____��
A��Fe(CO)5�ǷǼ��Է��ӣ�CO�Ǽ��Է���
B��Fe(CO)5��Feԭ����sp3�ӻ���ʽ��CO�ɼ�
C��1mol Fe(CO)5����10mol���
D����ӦFe(CO)5=Fe+5COû���»�ѧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ�����ֲ�����������彡������ʮ����Ҫ�����ã�Ҳ�㷺Ӧ�������Ͳ��ϵ��Ʊ���
(1)��̬��ԭ�ӵļ۵��ӹ������ʽ��_______________��������ͬ���������ڵ�����Ԫ�غ���ĵ�һ�������ɴ�С��˳��Ϊ___________��
(2)�������ʵĻ����ṹ��ԪΪ����ʮ���壬�����Է��س��ֶ��������Σ��������ʳ�Ϊ�����________��
(3)B�ļ��⻯��BH3����������ڣ����������γɽ��ȶ���B2H6�����������ӽ�ϡ�
��B2H6���ӽṹ��ͼ����Bԭ�ӵ��ӻ���ʽΪ________��

�ڰ�����(NH3BH3)����Ϊ�����DZ�������ʹ������֮һ�������д�����λ�����ṩ�µ��ӶԵijɼ�ԭ����______��д��һ���백���黥Ϊ�ȵ�����ķ���_____(�ѧʽ)��
(4)������(H3BO3)Ϊԭ�Ͽ��Ƶ����⻯��(NaBH4)�������л��ϳ��е���Ҫ��ԭ����BH![]() �ļ�����________�����幹��Ϊ___________��
�ļ�����________�����幹��Ϊ___________��
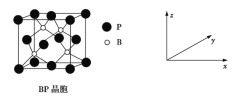
(5)����(BP)���ܸ߶ȹ�ע����ĥ���ϣ�����Ϊ��������ı����㣬��ṹ����ʯ���ƣ������ṹ��ͼ��ʾ��������z����ƽ���ͶӰͼ�У�Bԭ�ӹ��ɵļ�����״��_______����֪�����߳�Ϊ458 pm������������ܶ���____g��cm��3(��ʽ�����㣬���������λ��Ч���֣���֪4��583��96��07)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ڹ�ũҵ�������й㷺����;��
���������Ʊ������ܶࡣ
(1)�Ʊ�����һ��H2O2��Һ�������Ca(OH)2����Һ��Ӧ���Ʊ�CaO2��8H2O���仯ѧ����ʽΪ______________________________________________________________��
(2)�Ʊ������������÷�ӦCa(s)+O2![]() CaO2(s)���ڴ�����������ȡCaO2��ʵ����ģ��װ��ʾ��ͼ���£�
CaO2(s)���ڴ�����������ȡCaO2��ʵ����ģ��װ��ʾ��ͼ���£�
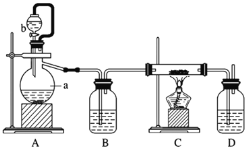
��ش��������⣺
��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ________________������a������Ϊ ___________��
��װ��D��ʢ�е�Һ����Ũ���ᣬ������һ��_________������_____________��
��ˮ�������г���ˮ�м�һ����CaO2��8H2O������������DO����ˮ����������DO������ÿ��ˮ���ܽ���������������ʾ����ⶨ���輰ԭ��Ϊ��
a�������������£�O2��Mn2+����ΪMnO(OH)2��2Mn2++O2+4OH=2MnO(OH)2����
b�������������£�MnO(OH)2��I����ΪI2��MnO(OH)2+2I+4H+=Mn2++I2+3H2O��
c���ζ�����Na2S2O3����Һ�ζ����ɵ�I2��2S2O32-+I2=S4O62-+2I��
ijͬѧ��ˮ�м�һ����CaO2��8H2O��ȡ��ˮ��100.00mL�������������ⶨˮ����������DO��������0.0100mol��L1 Na2S2O3����Һ13.50mL��
(1)�ζ�������ʹ�õ�ָʾ����_______________________________��
(2)��ˮ���е��ܽ�������DO��Ϊ__________________mg��L1��
(3)����b�м���������Һ��Ӧ������ҺpH���ͣ��ζ�ʱ��������Ե���д������������ԭ��________________________�������ӷ���ʽ��ʾ������д��2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ҵ�����Ϊ21���͵�����ɫ��Դ��������ú�����Ṥ�ճ��졢�ɱ�����������������Ҵ��нϴ�ļ۸���˿�չ����������Ҵ��Ĵ���Ӧ�о�������Ҫ���塣�ش���������:
��1������������Ҵ���һ�����ӵķ�Ӧ��ϵ����ͬ�ķ�Ӧ�����ᵼ���γɲ�ͬ�IJ�Ʒ�ֲ�����Ҫ��Ӧ��������:
��Ӧ��CH3COOH(g) + H2(g)��CH3CHO(g) +H2O(g) H1
��Ӧ��CH3CHO(g) +H2(g) ��CH3CH2OH(g) ��H2
���ָ���Ӧ����:
��Ӧ��CH3CH2OH(g) + CH3COOH(g)��CH3COOCH2CH3(g) +H2O(g) H3
��Ӧ��CH3COOCH2CH3(g) +2H2(g) ��2CH3CH2OH(g) H4
H4=___ (��H1����H2�� H3��ʾ)��
��2������������Ҵ��ķ�Ӧ�ɱ�ʾΪCH3COOH(g) + 2H2(g)==CH3CH2OH(g) + H2O(g) H<0������߸÷�Ӧ�������ƽ��ת���ʣ��ɲ�ȡ��������ʩ��_________���������������������( ����Ӧ)�Ļ�ѧ����ʽ�ɱ�ʾΪ____________.
��3���ں���2609��C�� ��ѹ1.4MPa, ԭ������һ��������ͨ��ij������������������Ҵ��ȷ�Ӧ����/��(���ʵ���)Ͷ�ϱȶԷ�Ӧ��Ӱ����ͼ��ʾ��X������ʾ�����ת����, S�Ҵ���S���������ֱ��ʾ�Ҵ�������������ѡ���ԣ����У�S�Ҵ�=![]() ��S��������=
��S��������=![]() ������ͼ�����ݷ����������/��Ͷ�ϱ�ֵ��_______________,�������£�������Ӧ��____________(������������������)���е��ף�������____________ ;����ͼ�����߱仯���ɣ��ж���/��Ͷ�ϱ�=30ʱ��S�����������������������ͼ��a��b��c�е�______��;��/��Ͷ�ϱ�=20ʱ������H2��ת����=_______. ( �г�����ʽ���ɣ���ͬ),�Ҵ��ķ�ѹP�Ҵ�=__________(��ѹ=��ѹ�����ʵ�������)��
������ͼ�����ݷ����������/��Ͷ�ϱ�ֵ��_______________,�������£�������Ӧ��____________(������������������)���е��ף�������____________ ;����ͼ�����߱仯���ɣ��ж���/��Ͷ�ϱ�=30ʱ��S�����������������������ͼ��a��b��c�е�______��;��/��Ͷ�ϱ�=20ʱ������H2��ת����=_______. ( �г�����ʽ���ɣ���ͬ),�Ҵ��ķ�ѹP�Ҵ�=__________(��ѹ=��ѹ�����ʵ�������)��
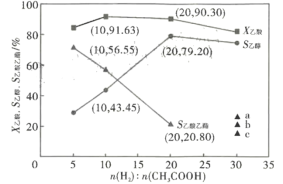
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A<B<C<D<E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�AC2�ǷǼ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2:1����������������硣��������������ش��������⣺(����ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
(1)A��B��C�ĵ�һ��������С�����˳��Ϊ_________��
(2)B���⻯��ķ������幹����_____��������ԭ�Ӳ�ȡ_______�ӻ���
(3)д��������AC2�ĵ���ʽΪ_______��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ______��
(4)E�ĺ�������Ų�ʽ��______��ECl3�γɵ������Ļ�ѧʽΪ_____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(AlN��Al��N�����ԭ�������ֱ�Ϊ27��14)�㷺���ڵ��ӡ��մɵȹ�ҵ������һ�������£�AlN��ͨ����ӦAl2O3+N2��3C![]() 2AlN+3CO�ϳɡ�����������ȷ����(����)
2AlN+3CO�ϳɡ�����������ȷ����(����)
A.������Ӧ�У�N2�ǻ�ԭ����Al2O3��������
B.������Ӧ�У�ÿ����1mol AlN��ת��3mol����
C.AlN�е�Ԫ�صĻ��ϼ�Ϊ+3
D.AlN��Ħ������Ϊ41g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˾ƥ��(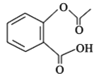 )��һ����;�ܹ��������ʹҩ�����ͨ�����·����ϳɣ�
)��һ����;�ܹ��������ʹҩ�����ͨ�����·����ϳɣ�

��֪��
![]() +
+![]()
![]()
![]() +HCl
+HCl
3CH3COOH+PCl3��3CH3COCl+H3PO3
��ش��������⣺
(1)��˾ƥ���еĺ�����������____(д����)����Ӧ�ٵĻ�ѧ��Ӧ����Ϊ____��
(2)ͨ��ˮ���ỹ���Ƶ�����E�߷��ӻ�����G��
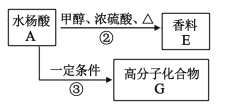
����д��A��E�Ļ�ѧ��Ӧ����ʽ____��
����֪G��һ�־�������д����ṹ��ʽ____��
(3)д��ˮ�����������ڷ�������ͬ���칹��Ľṹ��ʽ____��
(4)���������Ϣ���Ա��ӡ��춡ϩΪԭ��(������ԭ����ѡ)�ϳɾۺ��� ������·�����£�
������·�����£�![]()
![]() ������
������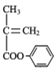 ��
�� �������ƺϳ�����ʡ�Բ���____��
�������ƺϳ�����ʡ�Բ���____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Ga)����(Ge)����(Si)����(Se)�ĵ��ʼ�ijЩ���������黯�ء����صȶ��dz��õİ뵼����ϣ�Ӧ���ں��պ����ء�����ͨѶ�����ش��������⣺
(1)���������������ϣ���̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]_______������ͬ���ڵ�p��Ԫ���е�һ�����ܴ�������Ԫ����_____�֣�SeO3�Ŀռ乹����_______��
(2)����Ԫ�������ɣ�ԭ�Ӱ뾶Ga ___As����һ������Ga _____As��(����ڡ���С�ڡ�)
(3)ˮ������Ҫ�ɷ��Ƕ������裬��ˮ���й�ԭ�ӵ���λ����______�������������γ�һϵ�еĶ�Ԫ������SiH4��Si2H6�ȣ����ȡ��������γ�SiCl4 ��SiBr4,�����������ʵķе��ɸߵ��͵�˳��Ϊ__________������ϩ(Si4H8)�ЦҼ���м�����֮��Ϊ___��
(4)GaN��GaP��GaAs���Ǻܺõİ뵼����ϣ����������뾧������ƣ��۵����±���ʾ��������仯ԭ��___��
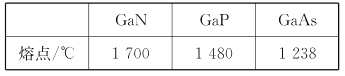
(5)GaN����ṹ��ͼ��ʾ����֪�������ױ߱߳�Ϊa cm�������ӵ�������ֵΪNA��
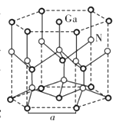
�پ�����Gaԭ�Ӳ����������ܶѻ���ʽ��ÿ��Gaԭ����Χ���������Gaԭ����ĿΪ_____��
�ڴ�GaN�����С��ָ����ƽ����������ͼ��ʾ������ƽ������������Ϊ![]() cm3��GaN������ܶ�Ϊ____g/cm3(��a��NA��ʾ)��
cm3��GaN������ܶ�Ϊ____g/cm3(��a��NA��ʾ)��
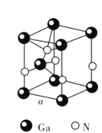
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com