


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
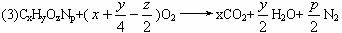
����������й����⣺
(1)ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������______________________��
(2)װ��ͼ����Ҫ���ȵ�������__________��(����ĸ��գ���ͬ)������ʱӦ�ȵ�ȼ__________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��______________________________________��
(4)Dװ�õ�������______________________________________________________��
(5)��ȡN2�����ʱ��Ӧע�⣺��___________;��___________��
(6)ʵ���в��N2�����ΪV mL(������Ϊ��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������___________ (����ĸ���)��
A.���ɶ�����̼��������� B.����ˮ������
C.ͨ����������� D.���������Է�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)ʵ�鿪ʼһ��ʱ��ɹ۲쵽��ƿ���Ϸ��а������ɣ��뽫���ܹ۲쵽������������___________���йػ�ѧ����ʽΪ____________________��
(2)�������µ�ԭ������ʵ��һ��ʱ�������̫���ԣ��������ڼ��������������ʲ��ܿ������Ե�����__________(�����)��
A.NaOH���� B.��ʯ�� C.ŨH2SO4 D.NH4Cl����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com