
���� ����ǰ10��Ԫ�صĵ����Ų����ɳɼ��ص���γɷ��ӵ�ԭ����Ŀ�Ƶ������ķ�����������ԭ�ӷ��ŵĺ�����⣮
��� �⣺��1���������֪��һ��ԭ���к���һ�����ӣ���ԭ��ΪH���ʴ�Ϊ��H2��
��2��1���������4�����ӵ�ԭ����̼ԭ�ӣ���2���������6�����ӵ�ԭ������ԭ����ϵķ�����CO2���ʴ�Ϊ��CO2��
��3���ɺ�������Ų����γɷ��ӵ�ԭ����Ŀ��֪��ԭ�ӷֱ���N��H���γɵķ���ΪNH3���ʴ�Ϊ��NH3��
��4���ɺ�������Ų����γɷ��ӵ�ԭ����Ŀ��֪��ԭ����O���γɵķ���ΪO3���ʴ�Ϊ��O3��
��5���ɺ�������Ų����γɷ��ӵ�ԭ����Ŀ��֪���÷���ΪHF���ɺ�������Ų����γɷ��ӵ�ԭ����Ŀ��֪���÷���ΪCH4���ʴ�Ϊ��HF��
CH4��
���� ���⿼����ӡ�ԭ�ӡ����ӣ�����ǰ10��Ԫ�صĵ����Ų�������ɳɼ��ص���γɷ��ӵ�ԭ����Ŀ�Ƶ������������Ĺؼ���


 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Һ̬HCl����̬NaCl�������磬����HCl��NaCl�Ƿǵ���� | |
| B�� | NH3��CO2��Cl2��ˮ��Һ���ܵ��磬����NH3��CO2��Cl2���ǵ���� | |
| C�� | ���ǡ��Ҵ���Һ̬��ˮ��Һ��������磬���������Ƿǵ���� | |
| D�� | �ռ�����ᡢʯī��Ϊ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 32g O2���е���ԭ����ĿΪNA | |
| B�� | ���³�ѹ�£�22.4L CO2���еķ�����ĿΪNA | |
| C�� | lmolNa��������ȫ��Ӧ����Na2O2ʱʧȥ�ĵ�����ĿΪNA | |
| D�� | 1mol/L K2CO3��Һ�к��еļ�������ĿΪ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
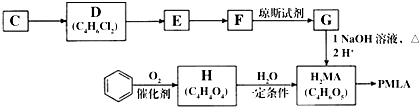


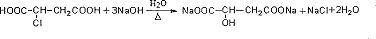
 �ȣ�
�ȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ | B�� | ��Ȳ | C�� | ���� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2NO��g��+O2��g���T2NO2��g�� | B�� | 2C2H6��g��+7O2��g���T4CO2��g��+6H2O��g�� | ||
| C�� | NH4NO3��s���TNH4+��aq��+NO3-��aq�� | D�� | H2��g��+Cl2��g���T2HCl��l�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
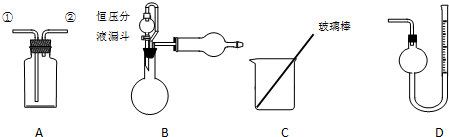
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
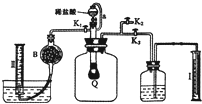 g]
g]�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com