��2013?����ģ�⣩��ˮAlCl
3�������л��ϳɵĴ�����ʳƷ���ɼ��ȣ���ҵ������������Ҫ�ɷ���Al
2O
3��Fe
2O
3����ʯ�ͽ�����Ҫ�ɷ���̼���ʣ��Ʊ���ˮAlCl
3���������£�

��1���ڱ���¯�з�����Ӧ��
��Fe
2O
3��s��+3C��s��?2Fe��s��+3CO��g����
��3CO��g��+Fe
2O
3��s��?2Fe��s��+3CO
2��g����
��Ӧ�ڵ�ƽ�ⳣ���ı���ʽΪK=
��
��2��Al
2O
3��Cl
2��C���Ȼ�¯�и����·�����Ӧ��������1mol AlCl
3ʱת��
3
3
mol���ӣ�¯���к��д���CO������Cl
2������Na
2SO
3��Һ��ȥCl
2�������ӷ���ʽΪ
SO32-+C12+H2O�TSO42-+2C1-+2H+
SO32-+C12+H2O�TSO42-+2C1-+2H+
��
��3���������з�����Ӧ�Ļ�ѧ����ʽΪ
��
��4����ҵ����һ��������Ϊԭ���Ʊ���ˮAlCl
3�����У����һ������AlCl
3?6H
2O��ȥ�ᾧˮ�Ʊ���ˮAlCl
3���˷�Ӧ�������Ȼ���������м��ȣ���ԭ����
����AlCl3��ˮ��
����AlCl3��ˮ��
��
��֪SOCl
2Ϊ��ɫҺ���Ҽ�����ˮ��Ӧ����HCl��SO
2��AlCl
3?6H2O��SOCl
2��ϼ��ȿ���ȡ��ˮAlCl
3��д���÷�Ӧ�Ļ�ѧ����ʽ��
AlCl
3?6H
2O+6SOCl
2AlCl
3+12HCl��+6SO
2��
AlCl
3?6H
2O+6SOCl
2AlCl
3+12HCl��+6SO
2��
��





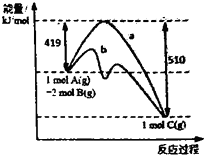 ��2013?����ģ�⣩��ӦA��g��+2B��g��=C��g���ķ�Ӧ�����������仯��ͼ��ʾ������a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾʹ�ô�����������仯���������˵����ȷ���ǣ�������
��2013?����ģ�⣩��ӦA��g��+2B��g��=C��g���ķ�Ӧ�����������仯��ͼ��ʾ������a��ʾ��ʹ�ô���ʱ��Ӧ�������仯������b��ʾʹ�ô�����������仯���������˵����ȷ���ǣ�������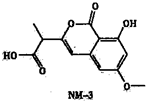 ��2013?����ģ�⣩NM-3�����������ٴ�����ε�С���ӿ���ҩ���ṹ��ͼ��ʾ��δ��ʾ����ռ乹�ͣ������й���NM-3������������ȷ���ǣ�������
��2013?����ģ�⣩NM-3�����������ٴ�����ε�С���ӿ���ҩ���ṹ��ͼ��ʾ��δ��ʾ����ռ乹�ͣ������й���NM-3������������ȷ���ǣ�������