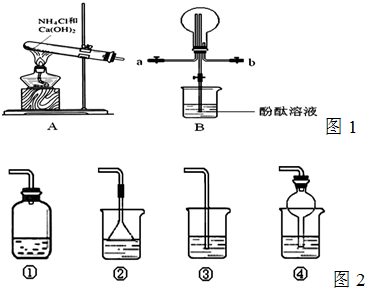
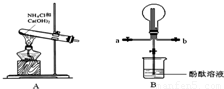
��ͼʵ�����Ʊ����������а�������ʵ���װ�ã�����ɣ�
��1��ʵ������ͼ���Ʊ������Ļ�ѧ����ʽΪ
��
��2��NH
3���ӵĿռ乹��Ϊ
��
��3��ͼ����ij��ѧ��ȤС���ͬѧ�Ʊ���������������ʵ��ʱ�ĸĽ�װ�ã���Ũ��ˮ�����Թ�������ɹ۲쵽�Թ��ڷ������ҷ�Ӧ���д������ݲ�����
��ͼ��������NH
3��Բ����ƿȡ�£���װ��ͼ����ʾ��װ�ã���ͷ�ι�����������2mLˮ����ʱС����ϵ�ڲ������ϳ���Ȼ�ɳ�״̬�����ι��ڵ�ˮ����������ƿ�У�����ζ���ƿ��ͨ���۲�ʵ������������֤NH
3��ij�����ʣ�
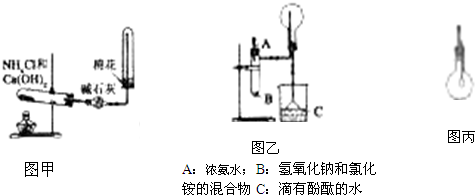
��������ijͬѧ���ڸû�ѧС������ͼ����ȡNH
3��ԭ����������Ͽ�ѧ��ԭ������
������ţ�
A����NH
3?H
2O����ƽ�⣺NH
3+H
2O?NH
3?H
2O?NH
4++OH
-��NaOHʹƽ�������ƶ�
B����NH
3?H
2O����ƽ�⣺NH
3+H
2O?NH
3?H
2O?NH
4++OH
-��NH
4Clʹƽ�������ƶ�
C��NaOH����ˮʱ���ȣ�ʹ��ϵ���¶����ߣ�NH
3���ܽ�ȼ�С�����в���NH
3�ų���
D��NH
4Cl��ֽ��ͷų�NH
3��ͼ���е�NH
4Cl��NaOH���������ܷ���CaO�������
����ܡ����ܡ�����
������ж�ͼ������ƿ������NH
3��
��
��ͼ�����潺ͷ�ι��е�ˮ������ƿ�У��۲쵽��������
��˵��NH
3
�����ʣ�




 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�