
����Ŀ���ش���������:
(1)��֪������CO��ȼ����Ϊ283kJ/mol����CO��ȼ���ȵ��Ȼ�ѧ����ʽΪ____��
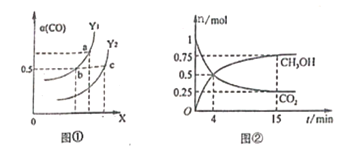
(2)��ҵ������CO��H2�ϳ������ԴCH3OH���䷴ӦΪ:CO(g)+2H2(g)![]() CH3OH(g) H=-116kJ/mol����ͼ�ٱ�ʾCO��ƽ��ת����(
CH3OH(g) H=-116kJ/mol����ͼ�ٱ�ʾCO��ƽ��ת����(![]() )���¶Ⱥ�ѹǿ�仯��ʾ��ͼ�к�����X��ʾ����_________��Y1______Y2(������������=����������)��
)���¶Ⱥ�ѹǿ�仯��ʾ��ͼ�к�����X��ʾ����_________��Y1______Y2(������������=����������)��
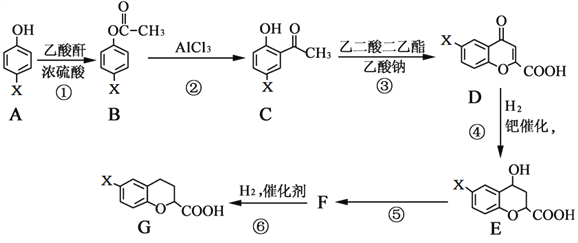
(3)�ϳɼ״��ķ�Ӧԭ��Ϊ:CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����0.5L���ܱ����У�����1 mol CO2��3molH2����500��������Ӧ�����CO2(g��CH3OH(g)������ʱ��仯��ͼ����ʾ��
CH3OH(g)+H2O(g)����0.5L���ܱ����У�����1 mol CO2��3molH2����500��������Ӧ�����CO2(g��CH3OH(g)������ʱ��仯��ͼ����ʾ��
�ٷ�Ӧ���е�4minʱ��v(��)____v(��)(������������=����������)��0��4min��H2��ƽ����Ӧ����v(H2)=_____________��
�ڸ��¶���ƽ�ⳣ��Ϊ_____________��
��������˵���÷�Ӧ�Ѵﵽƽ��״̬����___________��
A. v��(CH3OH)=3v��(H2)
B.CO2��H2��CH3OH��H2OŨ��֮��Ϊ1:3:1:1
C.���º�ѹ�£������������ٱ仯
D.���º����£�������ܶȲ��ٱ仯
(4)Ϊ���ȼ�ϵ����������ʣ����������Ϊȼ�ϵ�ء�ij����Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ���Ծ��д����ú͵������ܵ�ϡ������Ϊ�缫д����ȼ�ϵ�صĸ�����Ӧʽ��_________��
���𰸡�CO(g)+ ![]() O2(g) = CO2(g) ��H=-283kJ/mo1 ѹǿ �� �� 0.75 mol��L1��min1
O2(g) = CO2(g) ��H=-283kJ/mo1 ѹǿ �� �� 0.75 mol��L1��min1 ![]() C CH3OH - 6e- + 8 OH- = CO32-+6H2O
C CH3OH - 6e- + 8 OH- = CO32-+6H2O
��������
(1)����CO��ȼ����Ϊ283kJ/mo1�����ȼ���ȵĻ�ѧ����ʽ����дҪ����д��
(2)CO(g)+2H2(g)CH3OH(g)��H=-116kJ/mo1����Ӧ�����������С�ķ��ȷ�Ӧ��ͼ����CO ��ƽ��ת������X�����������ͬ������Y1��Y2������һ����̼ת����Y1��Y2������¶Ⱥ�ѹǿ��ƽ���Ӱ������жϣ�
(3)��ͼ�����4minʱ���״��Ͷ�����̼Ũ����ʱ�������仯����Ӧδ�ﵽƽ��״̬������ͼ���ȼ���v(CO2)���ٸ��ݷ���ʽ����v(H2)����15min��Ӧ�ﵽƽ��״̬������ƽ��ʱ�״������ʵ���Ϊ0.75mol���������ʽ����ƽ�ⳣ��K���۸������淴Ӧ������ͬ������ֺ������ֲ�������жϣ�
(4)��������������Ӧ���״�����������̼��أ��ݴ���д�缫��Ӧʽ��
(1)CO��ȼ����Ϊ283kJ/mo1����ȼ���ȵ��Ȼ�ѧ����ʽΪCO(g)+![]() O2(g)CO2(g)��H=-283kJ/mo1���ʴ�Ϊ��CO(g)+
O2(g)CO2(g)��H=-283kJ/mo1���ʴ�Ϊ��CO(g)+![]() O2(g)CO2(g)��H=-283kJ/mo1��
O2(g)CO2(g)��H=-283kJ/mo1��
(2)CO(g)+2H2(g)CH3OH(g)��H=-116kJ/mo1���÷�Ӧ�����������С�ķ��ȷ�Ӧ��ͼ����CO ��ƽ��ת������X�����������X ��ʾ����ѹǿ��Y��ʾ�����¶ȣ���ͬ�����£���Y1��Y2������һ����̼ת����Y1��Y2�������¶ȣ�ƽ�������ƶ������¶�Y1��Y2���ʴ�Ϊ��ѹǿ������
(3)�ٷ�Ӧ���е�4min ʱ����ʱ��仯���״����ӣ�������̼��С��˵����Ӧ����������У�v(��)��v(��)��0��4min��������̼���ʵ����仯1mol-0.5mol=0.5mol��CO2��ƽ����Ӧ����v(CO2)= =0.25mol/(Lmin)����v(H2)=3 v(CO2)=3��0.25mol/(Lmin)=0.75mol/(Lmin)���ʴ�Ϊ������0.75 mol/(Lmin)��
=0.25mol/(Lmin)����v(H2)=3 v(CO2)=3��0.25mol/(Lmin)=0.75mol/(Lmin)���ʴ�Ϊ������0.75 mol/(Lmin)��
��15min��Ӧ�ﵽƽ��״̬���״�ƽ��Ũ��Ϊ0.75mol/L��������̼ƽ��Ũ��Ϊ0.25mol/L��
CO2(g)+3H2(g)CH3OH(g)+H2O(g)
��ʼ��(mol) 1 3 0 0
�仯��(mol) 0.75 2.25 0.75 0.75
ƽ����(mol) 0.25 0.75 0.75 0.75
K= =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��A������֮�ȵ��ڻ�ѧ����ʽ�еĻ�ѧ������֮�ȣ�3v��(CH3
(4)ȼ�ϵ���У�ͨ��ȼ�ϵ�Ϊ��������������������Ӧ���״�����������̼��أ��缫����ʽΪCH3OH - 6e- + 8 OH- = CO32-+6H2O���ʴ�Ϊ��CH3OH - 6e- + 8 OH- = CO32-+6H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������ҩ�����Ѫ�����Ż��Ե�ѡ������1���������������������ںϳɸ�ҩ����м���G�IJ����������£�
��֪���������Ľṹ��ʽΪ��![]()
��ش��������⣺
��1��G�����еĺ��������ŵ�������________��________��
��2����ӦA��B�Ļ�ѧ����ʽΪ_________________________________________��
��3�������ܡ��ݱ仯���̵ķ�Ӧ���ͷֱ���_______________��______________��
��4���л���F�Ľṹ��ʽΪ______________________��
��5��д����������������C��ͬ���칹��Ľṹ��ʽ��___________��___________��
��. ������ֻ������ȡ������
��. ������ֻ��4�ֲ�ͬ��ѧ�������⡣
��. ����NaHCO3��Ӧ����CO2��
��6����������֪ʶ����������Ϣ�����������![]() ��������Ϊԭ���Ʊ�
��������Ϊԭ���Ʊ�![]() �ĺϳ�·������ͼ�����Լ���ѡ�������ֺϳ�·������ͼ���£�
�ĺϳ�·������ͼ�����Լ���ѡ�������ֺϳ�·������ͼ���£�

����ɺϳ�·��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��R��X��Y��Z��ԭ��������������Y��Zԭ������������֮�͵���Xԭ��������������2����R��Yλ��ͬ���塣R��X��Z����һ�ַ��ӵĽṹʽ��ͼ��ʾ������˵��������ǣ� ��
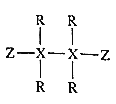
A.ԭ�Ӱ뾶��Y��Z��X��R
B.YR��YZ�������ӻ�����
C.Z���������Ӧ��ˮ������ǿ��
D.���³�ѹ�£�X���⻯����ܳ�Һ̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������M(C16H14O2)��һ�����ϣ���ҵ��������A�ͼױ�Ϊ��Ҫԭ�ϣ�������·�ߺϳɣ�

��֪������̬��A�ڱ�״���µ��ܶ���1.25g/L������Ϊ��ʵ���������ȩ�ڼ�����Һ���ܷ�����ȩ���Ϸ�Ӧ������ˮ���ɲ�����ȩ��RCH2CHO+ 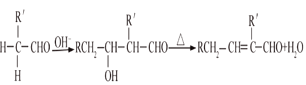 ����ش��������⣺
����ش��������⣺
��1��K��������___________��G�к��еĹ�����������______________��
��2��д��D��E�Ļ�ѧ����ʽ____________________________________��
��3��A��B�ķ�Ӧ������_______��
��4��ͬʱ������������������K��ͬ���칹����_____��(�����������칹)������FeCl3��Һ������ɫ��Ӧ�����ܷ���������Ӧ�������������ⲻ�ٺ���������״�ṹ�����к˴Ź�������Ϊ�����Ľṹ��ʽΪ____��
��5�����Ҵ�Ϊ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ���ƺϳ�1-��ϩ��·�ߡ����ýṹ��ʽ��ʾ�л����ͷ��ע���Լ��ͷ�Ӧ������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����Ӿ�������ݴ�С�Ƚϲ���ȷ����(����)
A. �����ܣ�NaF>NaCl>NaBr
B. Ӳ�ȣ�MgO>CaO>BaO
C. �۵㣺NaF>MgF2>AlF3
D. �����ӵ���λ����CsCl>NaCl>CaF2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ҫ����ԴҲ����Ҫ�Ļ���ԭ�ϡ�
��֪��ӦCH4(g)+2NO2(g)![]() N2(g)+CO2(g)+2H2O(g)����ʼʱ�����ΪV�ĺ����ܱ�������ͨ��2molCH4��3molNO2�����CH4��N2��H2O�����ʵ���Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
N2(g)+CO2(g)+2H2O(g)����ʼʱ�����ΪV�ĺ����ܱ�������ͨ��2molCH4��3molNO2�����CH4��N2��H2O�����ʵ���Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
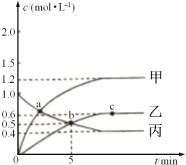
��1���������V=_____L��
��2��ͼ�б�ʾH2O�����ʵ���Ũ����ʱ���ϵ��������_____(������������"��"��")��
��3��0~5min�ڣ���N2��ʾ�Ļ�ѧ��Ӧ����Ϊ_____mol��L-1��min-1��
��4��a��b��c�����дﵽƽ��ĵ���______���ﵽƽ��ʱ��NO2��ת������_____��(����ƽ��ת����=ת�������ʵ���/��ʼ�����ʵ�����100%)��
��5��a��ʱ��n(CH4):n(NO2)=_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£�A��B��C���������ں����ܱ������н��з�Ӧ����Ӧ��0��2min���й����У��ڲ�ͬ��Ӧʱ������ʵ����ı仯�����ͼ��ʾ��
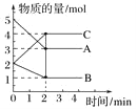
��1���÷�Ӧ�ķ�Ӧ����____________����������________��
��2����ѧ����ʽΪ___________________________________________��
��3���ܷ���㷴Ӧ��ʼ��2minʱ����C��ʾ�ķ�Ӧ���ʣ�________�������ܣ�����ԭ����_________________________________________________��
��4�����ڸ÷�Ӧ��˵����ȷ����________(����ĸ����ͬ)��
a������2minʱ����Ӧֹͣ
b����2min֮ǰA���������ʴ���A����������
c����2minʱ�ﵽƽ��״̬����Ϊ��ʱ��Ӧ�������ʵ����������������ʵ������
d��2minʱ����Ӧ�������淴Ӧ�������
��5�����п��жϷ�Ӧ�Ѵﵽƽ��״̬����________��
a��A��B��C�ķ�Ӧ�������
b��A��B�ķ�Ӧ����֮��Ϊ2��1
c����������ѹǿ����
d������1molC��ͬʱ����1molA��0.5molB
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϊ����һ�������������仯�������������������й㷺��;��
��1����ԭ�Ӻ�����ӷ���ԾǨʱ�����ջ��ͷŲ�ͬ�Ĺ⣬������___��ȡ��Ԫ�ص�ԭ�ӹ��ס�
��2��FeC13���۵�Ϊ306�棬�е�Ϊ315�档�ɴ˿�֪FeC13����____���塣FeSO4������ˮ���Ͳ�������SO42-�����幹����____��
��3�����軯�� K3[Fe(CN)6]�Ǽ���Fe2+����Ҫ�Լ���
�ٻ�̬Nԭ�ӵĹ����ʾʽΪ____��
��д��һ�������軯�������廥Ϊ�ȵ�����ļ��Է��ӵĻ�ѧʽ_____��
�����軯���У����漰��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ____��
�����軯���У�������___(����ĸ���)��
A�����Ӽ� B������ C������ D����� E��������
��4���л�������λ�������ï��[(C5H5)2Fe]�������еĿ�����������еĴ��������÷���![]() ��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�����(�籽�����еĴ������ɱ�ʾΪ
��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�����(�籽�����еĴ������ɱ�ʾΪ![]() )����
)����![]() �еĴ�����Ӧ��ʾΪ____������̼ԭ�ӵ��ӻ���ʽΪ____��
�еĴ�����Ӧ��ʾΪ____������̼ԭ�ӵ��ӻ���ʽΪ____��
��5���ʻ���[Fe(CO)5]���������������Ϳ������ȡ�1molFe(CO)5�����к�__mol��λ����
��6��ij�ִ��Ե������Ľṹ��ͼ��ʾ��N���������Fe���ɵ����������϶�С�������ԭ����Χ�������ԭ�Ӹ���Ϊ___���������ױ߳�Ϊacm����Ϊc cm�������ӵ�������ֵΪNA����ô��Ե������ľ����ܶ�Ϊ____g/cm3���г�����ʽ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com