

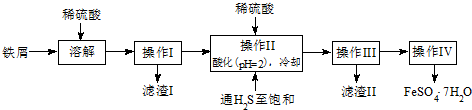
���� �������̷�������п�ϴ����г�������ZnO�⣬ͬʱ�����ܺ���CuO��Al2O3��Fe2O3���ʣ�������������ɢ����ȡ��пԪ��������[Zn��NH3��4]CO3�����˺���Һ�м���п�����û���Ӧ��п���Һ��п���Һ�������������յõ���ʽ̼��п������
��1��ZnO��Al2O3��������ǿ�����ZnO���������Ʒ�Ӧ����Na2ZnO2�Ļ�ѧ����ʽ���м��㣻
��2����ȡʱ����[Zn��NH3��4]CO3��ʱ��Ӧ��ΪZnO��NH3��NH4HCO3������Ԫ���غ���д��Ӧ�Ļ�ѧ����ʽ��
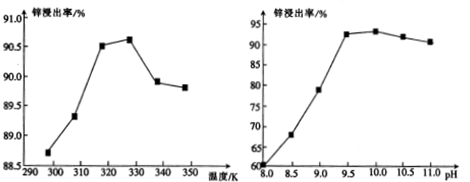
��3���ٸ����¶���п�����ʵ�ͼѡȡ��������ߵ�Ϊ�����¶ȣ��ڽ�ȡ�������¶ȹ��߰����ܽ���½���ͬʱ̼����炙�ֽ⣻
�ڸ���pH��п�����ʵ�ͼѡȡ��������ߵ�Ϊ����pHֵ��pH���ߣ����������ܽ⣬�Ӷ�����ƫ������������ʣ�
��4����ϴ�ӳ���ʱ�����ڹ������м�������ˮ������ͬ���ˣ��ظ�2��3�Σ�
��3.23gZnx��OH��y��CO3��z�����������������ٸı䣬���ɵ�ˮΪ0.36g��������̼Ϊ0.44g��ʣ���������п������Ϊ2.43g������Ԫ���غ�����п���⡢̼Ԫ�ص����ʵ���֮�ȣ�����ȷ��x��y��z�ı�ֵ����ʽ̼��п�Ļ�ѧʽ��
�۸��ݲ�������������ݣ�����Ԫ���غ��д��������Ӧ�Ļ�ѧ����ʽ��
��� �⣺�������̷�������п�ϴ����г�������ZnO�⣬ͬʱ�����ܺ���CuO��Al2O3��Fe2O3���ʣ�������������ɢ����ȡ��пԪ��������[Zn��NH3��4]CO3�����˺���Һ�м���п�����û���Ӧ��п���Һ��п���Һ�������������յõ���ʽ̼��п������
��1��ZnO��Al2O3��������ǿ�ZnO���������Ʒ�Ӧ����Na2ZnO2����Ӧ�Ļ�ѧ����ʽΪ2NaOH+ZnO�TNa2ZnO2+H2O�����ݷ���ʽ��֪1molZnO������2molNaOH����NaOH��Һ�����Ϊ4L��
�ʴ�Ϊ��4��
��2����ȡʱ����[Zn��NH3��4]CO3��ʱ��Ӧ��ΪZnO��NH3��NH4HCO3����Ӧ�Ļ�ѧ����ʽΪZnO+3NH3+NH4HCO3=[Zn��NH3��4]CO3+H2O��
�ʴ�Ϊ��ZnO+3NH3+NH4HCO3=[Zn��NH3��4]CO3+H2O��
��3���ٸ����¶���п�����ʵ�ͼ��֪��328K����ʱ��������ߣ��ڽ�ȡ�������¶ȹ��߰����ܽ���½���ͬʱ̼����炙�ֽ⣬����������[Zn��NH3��4]CO3��
�ʴ�Ϊ��328K�������ܽ���½���ͬʱ̼����炙�ֽ⣬����������[Zn��NH3��4]CO3��
�ڸ���pH��п�����ʵ�ͼ��֪��pHֵΪ9.5ʱ��������ߣ�pH���ߣ����������ܽ⣬�Ӷ�����ƫ������������ʣ���Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��9.5��Al2O3+2OH-=2AlO2-+H2O��
��4����ϴ�ӳ���ʱ���ز�������������м�������ˮ����û����Ϊֹ����ˮ�������ظ�2��3�Σ�
�ʴ�Ϊ���ز�������������м�������ˮ����û����Ϊֹ����ˮ�������ظ�2��3�Σ�
��3.23gZnx��OH��y��CO3��z�����������������ٸı䣬���ɵ�ˮΪ0.36g��0.02mol��������̼Ϊ0.44g��0.01mol��ʣ���������п������Ϊ2.43g��0.03mol������Ԫ���غ��֪��п���⡢̼Ԫ�ص����ʵ���֮��Ϊ3��4��1�����Լ�ʽ̼��п�Ļ�ѧʽΪZn3��OH��4CO3��
�ʴ�Ϊ��Zn3��OH��4CO3��
�۸��ݲ�������������ݿ�֪�����ɵĹ���ΪZn3��OH��4CO3������������Ӧ�Ļ�ѧ����ʽΪ3[Zn��NH3��4]CO3+2H2O$\frac{\underline{\;\;��\;\;}}{\;}$Zn3��OH��4CO3+12NH3��+2CO2����
�ʴ�Ϊ��3[Zn��NH3��4]CO3+2H2O$\frac{\underline{\;\;��\;\;}}{\;}$Zn3��OH��4CO3+12NH3��+2CO2����
���� ���⿼��ѧ�����ڹ�������ԭ�������⡢�Բ�����ʵ���������Ƶ�����ȣ��漰��������ͭ��Ԫ�ػ�����֪ʶ���Լ����û�ѧ������д�������ᴿ�ȣ���Ҫѧ���߱���ʵ�Ļ������ۺ������������Ѷ��еȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������������ѧʵ�顢��ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��Fe2+��Cl-��SO42- | B�� | K+��[Al��OH��4]-��Cl-��SO42- | ||
| C�� | Ca2+��Mg2+��NO3-��HCO${\;}_{3}^{-}$ | D�� | Na+��Cl-��CO32-��SO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Ʒ����Һ����ɫ��ȥ�����ȸ���ɫ��Һ������ɫ����������Ư���� | |
| B�� | ��ɫʯ����Һ���ȱ�죬����ɫ��������ˮ��Ӧ��������ʹ����� | |
| C�� | ����̪������������Һ����ɫ��ȥ������ֻ�������� | |
| D�� | ������Һ����Һ����ǣ�����ֻ����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ƽ�������������� | B�� | V������V����С | ||
| C�� | A��ת����� | D�� | ��Ӧ�ų�������������ԭ����2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4��C10 H22 | B�� | 1-��ϩ�ͻ����� | C�� | C2H4��1-��ϩ | D�� | �Ҷ����ͱ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com