
���� | NaF | NaCl | NaBr | NaI |
�۵�/�� | 995 | 801 | 755 | 651 |
���� | NaCl | KCl | RbCl | CsCl |
�۵�/�� | 801 | 776 | 715 | 646 |
���� | SiF4 | SiCl4 | SiBr4 | SiI4 |
�۵�/�� | -90.4 | -70.4 | 5.2 | 120 |
���� | SiCl4 | GeCl4 | SbCl4 | PbCl4 |
�۵�/�� | -70.4 | -49.5 | -36.2 | -15 |
(1)�Ƶ�±���P��������Ȼ�����۵���±���Ӽ���������ӵ�____________�йأ�����____________�������۵����ν��͡�
(2)���±���P�衢�ࡢ����Ǧ���Ȼ����۵���______________�йأ�����_______________����________________�����۵��������ߡ�
(3)�Ƶ�±������۵����Ӧ�Ĺ��±������۵�ߺܶ࣬����______________�йأ���Ϊһ��______________��______________�۵�ߡ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���������ֻ�ѧ�ս̰� �ս̰� ���ͣ�022
�ο��±�������й����⣺
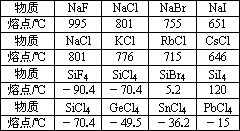
(1)�Ƶ�±���P��������Ȼ�����۵���±���Ӽ���������ӵ�________�йأ�����________�������۵����ν��ͣ�
(2)���±���P�衢�ࡢ����Ǧ���Ȼ����۵���________�йأ�����________����________�����۵��������ߣ�
(3)�Ƶ�±������۵����Ӧ�Ĺ��±������۵�ߺܶ࣬����________�йأ���Ϊһ��________��________�۵�ߣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���ɱ�������___________��һ�������ȼ�ϡ�
��2���Լ�������������Դ����Ҫ�ŵ���������Щ���棿
���ֳ���ȼ�ϵ���ֵ��
ȼ����Ҫ�ɷ� | ��ֵ/kJ��mol-1 |
�ƾ� | 1 367 |
��̿(C) | 300 |
��Ȼ��(��Ҫ��CH4) | 896 |
���� | 286 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
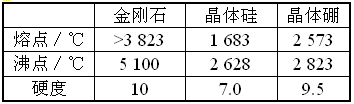

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
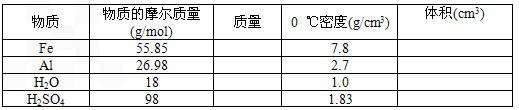
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com