
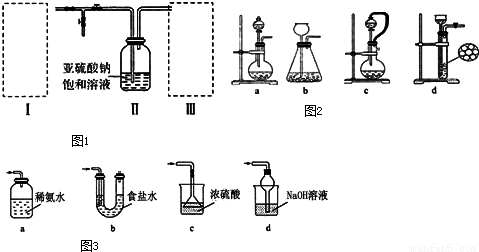
��10�֣����������ƣ�Na2S2O5��Ϊ��ɫ���ɫ�ᾧ��ĩ��С�ᾧ�������ʻ��ã�����ǿ��ԭ�ԣ���ʳƷ�ӹ�������������Ư�������ɼ���ijʵ��С���������ͼ1װ�ã�ʵ��ǰ�ѳ���װ���ڵĿ���������ȡ���������ƣ�Na2S2O5����
��1��װ��I�����������ƹ����Ũ�����Ʊ������������壬��װ���з�Ӧ�Ļ�ѧ����ʽΪ ���������Ʒ�Ӧ�ٶȣ���ͼ2�п�ѡ�õķ���װ���� ����д��ĸ����
��2��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ��2 NaHSO3=Na2S2O5 + H2O������Na2S2O5����������Ҫ����������ľ���ɲ�ȡ�ķ��뷽���� ��ijͬѧ��Ҫ420mL0.1mol/L������������Һ���о������ʣ�����ʱ��������������Ƶ�����Ϊ ������ʱ���õ�������ƽ��ҩ�ס��ձ����������������⣬�������õ��IJ��������� ��
��3��װ�â����ڴ���β������ѡ����ͼ3�������װ�ã��г���������ȥ��Ϊ ������ţ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ��ʯ�и�һ��ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ������
����m gij���壬����˫ԭ�ӷ��ӹ��ɣ�����Ħ������ΪM g / mol���������ӵ�������ֵ��NA��ʾ����ע����д��λ��
��1������������ʵ���Ϊ______________��
��2������������ԭ������Ϊ________________��
��3���������ڱ�״���µ����Ϊ__________________��
��4������������1 Lˮ��(�����Ƿ�Ӧ)������Һ�����ʵ���������Ϊ_ ______ __��
��5������������ˮ���γ�V L��Һ������Һ�����ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
��15�֣�������������X��Y��Z��M��N�����ǵ��� ���ӿ�����SO42-��Cl����NO3-��CO32-�������ӿ�����Ag����NH4+��Na����Al3����Cu2����Ba2����Fe3������֪��
���ӿ�����SO42-��Cl����NO3-��CO32-�������ӿ�����Ag����NH4+��Na����Al3����Cu2����Ba2����Fe3������֪��
��M����ɫ��Ӧ�ʻ�ɫ��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��X����Һ�����ԣ�Y��Z��N����Һ�����ԣ�M����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��X��Z����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��N��Z����Һ�����ɳ����������Ӱ�ˮ��Z�г�����ʧ��
�ް�X����Һ�ֱ���뵽Y��Z��N����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⣺
��1���������У�һ�������е���������_________��������������ͬ�������εĻ�ѧʽ��_____________��
��2��M�Ļ�ѧʽΪ_______________��M��Һ�Լ��Ե�ԭ����________________(�����ӷ���ʽ��ʾ)��
��3��X��Z����Һ��Ӧ�����ӷ���ʽ��______________��N�Ͱ�ˮ��Ӧ�����ӷ���ʽ��__________________��
��4����Ҫ����Y�������������ӣ���ȷ��ʵ�鷽����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�߶���ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ڿ��淴ӦA(g)��3B(s) 2C(g)��2D(g)���ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ�ķ�Ӧ����������
2C(g)��2D(g)���ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ�ķ�Ӧ����������
A��v(A)��0.5 mol��L��1��min��1 B��v(B)��1.2 mol��L��1��s��1
C��v(D)��0.4 mol��L��1��min��1 D��v(C)��0.1 mol��L��1��s��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�츣��ʡ������ѧ��10�µ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��A��B������ȫ��ͬ��װ�ã�ijѧ���ֱ������ǵIJ����װ��1.06 g Na2CO3��0.84gNaHCO3��A��B�зֱ���10 mL��ͬŨ�ȵ����ᣬ����������е�����ͬʱ������Ե��Թ���

����������ȷ����
A��Aװ�õ������������ʴ�
B�������������������ͬ���������Ũ��һ�����ڻ����2 mol/L
C�������������������ͬ���������Ũ��һ��С�ڻ����1 mol/L
D���������Թ���Na+��Cl-�����ʵ���һ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ��һ�ϵ�һ��������ѧ�Ծ��������棩 ���ͣ������
��6�֣�һ����������������ȼ�գ����û��������100 mL 2.00 moL��L-1NaOH��Һǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0.05mol���Լ����������⣺
��1��������Һ��Cl-�����ʵ���Ϊ ��
��2�����������Ͳμӷ�Ӧ�����������ʵ���֮��n(C12)��n(H2)Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ��ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵ�����ͬ����������ᣨH3PO4��������˵������ȷ����
A��ԭ����������ͬ B����ԭ������ͬ
C����������ͬ D����ԭ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ�����и�����ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����һ������ˮ��Һ�����ܴ����������������е������֣�K+��NH4+��Cl����Mg2+��Ba2+��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04mol
��3�������ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��
��������ʵ�飬�������ԭ��Һ���Ʋ���ȷ����
A��Cl��һ�������� B��K+һ������
C��Mg2+һ������ D��Ba2+���ܴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�찲��ʡ�����и�����ѧ�ڵ�һ���ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
���������ӡ��⡱ʳ�ν϶��ʹ���˵���أ�KIO3����������ڹ�ҵ�Ͽ��õ�ⷨ��ȡ����ʯī�Ͳ����Ϊ�缫����KI��ҺΪ���Һ����һ�������µ�⣬��Ӧ����ʽΪ��KI+3H2O==KIO3+3H2���������й�˵����ȷ����
A�����ʱ��ʯī�������������������
B�����ʱ��������Ӧ�ǣ�I--6e-+3H2O=IO-3+6H+
C����Һ������ǿ���ԣ�����������
D������������Һ��pH��С
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com