
��ͼΪ��ѧ�������ʼ��ת����ϵ�����мס��ҡ�������Ϊ���ʣ�����Ϊ�������������Ԫ������������������������C������Ϊ��ɫҺ�壬B��ɫ��ӦΪ��ɫ��һЩ����Һ�н��еķ�Ӧ��Һ�е�H2O�����ɵ�H2O��ʡ�ԡ�
�ش��������⣺
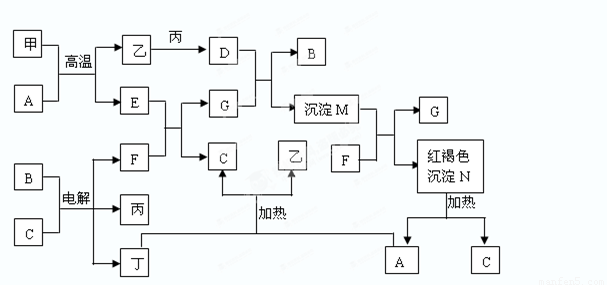
��1������������Ԫ�������ڱ��е�λ��______________________��
��2��������F�ĵ���ʽ________________________��
��3��D��G��Ӧ�����ӷ���ʽ___________________________________��
��4����A��Ӧ�Ļ�ѧ����ʽ___________________________________�����෴Ӧһ�㱻����________��Ӧ���÷�Ӧ����ҪӦ��_________________��(2��)
��1���������ڣ����� (2��) (дһ�벻����)
��2��
��3��Fe3����3AlO2����6H2O��Fe(OH)3����3Al(OH)3�� (2��) (��ѧʽ1�֣���ƽ1��)
��4��2Al��Fe2O3  Al2O3��2Fe (2��) (��������1��)
���ȡ�(2��)�����Ӹֹ졡ұ�����۽��� (2�֣�ÿ��1��)
Al2O3��2Fe (2��) (��������1��)
���ȡ�(2��)�����Ӹֹ졡ұ�����۽��� (2�֣�ÿ��1��)
��������
����������ס��ҡ�������Ϊ���ʣ�����Ϊ�������������Ԫ��������������������������Ϊ����C������Ϊ��ɫҺ�壬CΪˮ��NΪ���ɫ���壬NΪFe��OH��3��Fe��OH��3���ȷֽ�������������ˮ����AΪ�����������ת����ϵͼ��������ʵ����ʣ�����֪����Ϊ������Ϊ������Ϊ��������Ϊ������AΪ��������BΪ�Ȼ��ƣ� CΪˮ��DΪ�Ȼ�����EΪ��������FΪ�������ƣ�GΪƫ�����ƣ�MΪ�������������������Ļ�����1��������Ϊ��������Ԫ�������ڱ��е�λ�õ������ڣ����壻��2��������FΪ�������ƣ�����ʽΪ ����3���Ȼ�����Һ��ƫ��������Һ��Ӧ�����ӷ���ʽΪFe3����3AlO2����6H2O��Fe(OH)3����3Al(OH)3������4��������������Ӧ�Ļ�ѧ����ʽΪ2Al��Fe2O3 �� Al2O3��2Fe �����෴Ӧһ�㱻�������ȷ�Ӧ���÷�Ӧ����ҪӦ�ú��Ӹֹ졡ұ�����۽�����
���㣺��������ͼ�ƶϼ��������仯��������ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ���һ�и�����һ���¿���ѧ�Ծ� ���ͣ������
��12�֣���2011��ģ�⣩��ͼ����ѧ�������ʼ��ת����ϵ����֪��
a��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ�
b��EΪ����������JΪ���ɫ������
c��G��ʵ�����г����ڼ���B�Ĵ��ڣ�
d��L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ���ʻ�ɫ��
�ش��������⣺
��1��A�ĵ���ʽ ��
��2����Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������һ���¿���ѧ�Ծ� ���ͣ������
��12�֣���2011��ģ�⣩��ͼ����ѧ�������ʼ��ת����ϵ����֪��
a��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ�
b��EΪ����������JΪ���ɫ������
c��G��ʵ�����г����ڼ���B�Ĵ��ڣ�
d��L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ���ʻ�ɫ��
�ش��������⣺
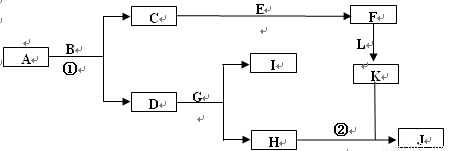
��1��A�ĵ���ʽ ��
��2����Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ������ѧ���ڳ����Ի�ѧ�Ծ� ���ͣ������
��12�֣���ͼ����ѧ�������ʼ��ת����ϵ����֪��
��AΪ����ɫ���壬BΪ���¡�����ЧӦ������Ҫ���ʣ� ��EΪ����������JΪ���ɫ������
��G��ʵ�����г����ڼ���B�Ĵ��ڣ���L��һ����Ҫ�Ĺ�ҵԭ�ϣ�����������ըҩ��Ũ��Һ���ʻ�ɫ����������ɫƿ�С�
�ش��������⣺
��1��A�ĵ���ʽ____________________________________________________��
��2����Ӧ�ٵĻ�ѧ����ʽΪ ����Ӧ�ڵ����ӷ���ʽΪ ��
��3�����μӷ�Ӧ��A������Ϊ39g��������CO2�����������£�Ϊ L��
��4��L�Ļ�ѧʽ ��G�Ļ�ѧʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com