
| A��ʵ�����У�Ũ���ᱣ���ڴ���������ɫϸ���Լ����У� |
| B���Ʊ�������������ʱ��Ӧ��20mL��ˮ����εμ�1~2mL���͵�FeCl3��Һ�����������ȵ�Һ������ĺ��ɫΪֹ�� |
C����ʯ�͵ķ���ʵ���У��¶� �������Һ���У� �������Һ���У� |
| D��������Ũ��Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ��������Һ�� |
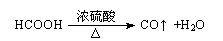 ��
��
 ____________________________��
____________________________��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���˵�����ɣ� ��
���˵�����ɣ� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
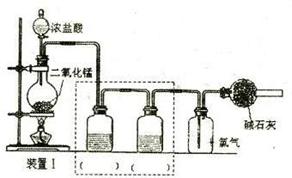
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
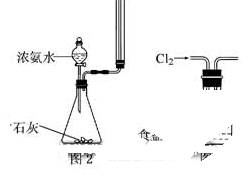
| A����ˮʪ��pH��ֽ����ij��Һ��pH |
| B������Ͳ��ȡ20mol ?L-1H2SO4��Һ���ձ��У���ˮ80mL�����Ƴ�0.1000 mol?L-1H2SO4��Һ |
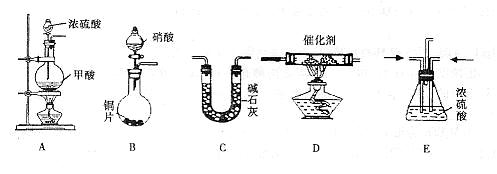
| C��ʵ������ͼ2��ʾװ����ȡ�������� |
| D��ʵ������ͼ3��ʾװ�ó�ȥCl2�е�����HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ʊ���Һ��NaOH�л���Na2CO3���� |
| B��ʢ��δ֪Һ����ƿ���ּ�������������ˮ |
| C���ζ�ǰ����ʱ���ӣ��ζ����յ����ʱ���� |
| D���ζ�����Һ��dz��ɫʱ����ֹͣ�ζ������ָֻ�����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �ܶ�Ϊ1.84 g��mL-1��_____________mL��
�ܶ�Ϊ1.84 g��mL-1��_____________mL�� ��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£�
��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£� �����ո����Բ��ӣ���
�����ո����Բ��ӣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
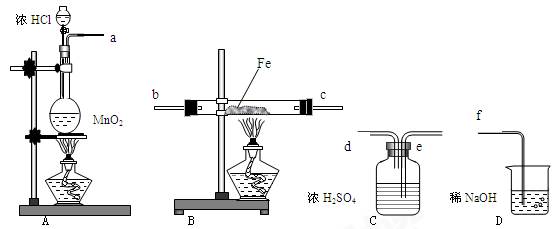
 ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���
ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У���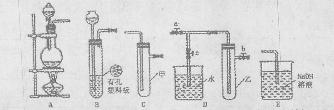
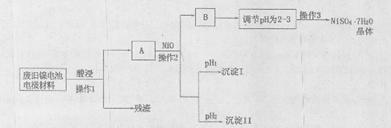
| M��OH��n | Ksp[�� | pH | |
| ��ʼ���� | ������ȫ | ||
| Al(OH)3 | 1.9��10-23 | 3.43 | 4.19 |
| Fe(OH)3 | 3.8��10-38 | 2.53 | 2.94 |
| Ni(OH)2 | 1.6��10-14 | 7.60 | 9.75 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com