
������ ����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ��������
����ҩ���ƹ����з��ֵ�һ����Ҫ�������ʣ����п����ʡ���������������ȱѪ�����ʡ�������ij�о�С�������һ�������� �����H�ĺϳ�·�ߣ�
�����H�ĺϳ�·�ߣ�
��1��ԭ��A��ͬ���칹���У����б������Һ˴Ź�����������4������� ��д����ṹ��ʽ����
��2����Ӧ�ڵĻ�ѧ����ʽ��_________________��
��3���۵ķ�Ӧ������___________��ԭ��D�к��еĹ�����������_________��_________��
��4��ԭ��B�������������������������ᣨ˳��ϩ��� ����������
����������
�����Ծ����б仯�ֱ�õ�ƻ���ᣨ ���;ۺ���Q��
���;ۺ���Q��
д����ӦI�ͷ�ӦII�Ļ�ѧ����ʽ��___________________��_____________________��
��5�����������������м����F��ͬ���칹����Ŀ��________�������������칹����д����������һ�ֵĽṹ��ʽ___________��
(i) �ܷ���������Ӧ��
(ii) �����к�����ȡ���ı����ṹ����������ȡ�����ǣ���COOCH3�� ���Ҷ��ߴ��ڶ�λ��
���Ҷ��ߴ��ڶ�λ��
����15�֣� ��
��1��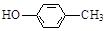
��2��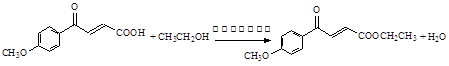
��3���ӳɷ�Ӧ��1�֣� ������1�֣� ���ӣ��ǻ���1�֣�
��4��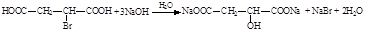
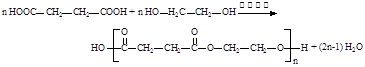
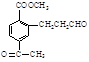
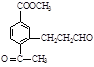
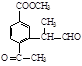
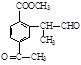 ������дһ�֣�
������дһ�֣�
�������������
��1��A�к����Ѽ����Ѽ���-OH���ڹ������칹���������ֵ�Ч�����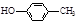 ��
��
��2����Ӧ�������м����E���м����F��-COOH����������Ӧת��Ϊ���������Է�ӦΪ��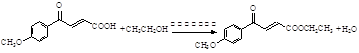 ��
��
��3����Ӧ�����п��Կ������м����F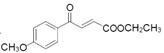 ��ԭ��D
��ԭ��D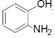 �����ӳɷ�Ӧ���м����G
�����ӳɷ�Ӧ���м����G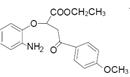 ��ԭ��D
��ԭ��D �к��а����ͣ��ӣ��ǻ���
�к��а����ͣ��ӣ��ǻ���
��4��������ͼ���Կ���������ֱ���HBr��H2�����ӳɷ�Ӧ����MΪ��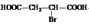 ����NaOH�з���ˮ�ⷴӦ��RΪ��
����NaOH�з���ˮ�ⷴӦ��RΪ��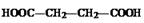 �����Ҷ����������۷�Ӧ��
�����Ҷ����������۷�Ӧ��
��5���м����F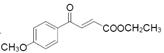 ��ͬ���칹�壬(i) �ܷ���������Ӧ������-CHO��
��ͬ���칹�壬(i) �ܷ���������Ӧ������-CHO��
(ii) �����к�����ȡ���ı����ṹ����������ȡ�����ǣ���COOCH3��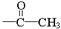 ���Ҷ��ߴ��ڶ�λ����һ��ȡ��������3��C����
���Ҷ��ߴ��ڶ�λ����һ��ȡ��������3��C����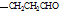 ��
��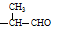 ���ֽṹ���ɵô𰸡�
���ֽṹ���ɵô𰸡�
���㣺���⿼�����л��������ƶϡ��ṹ�����ʡ�ͬ���칹֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ƽ�����ھ��������ƣ���ṹΪ��
(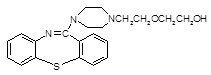 )2��
)2��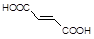 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�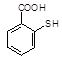

��֪������Ӧ��Ϊȡ����Ӧ������A��ϵͳ����Ϊ1,4������D2�D��ϩ��
��ش��������⣺
��1����Ӧ�ٵIJ���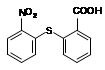 �г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
��2����Ӧ�۵������� ��Ӧ����Ӧ�ݵ�Ŀ���� ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4������B��ͬ���칹���ж��֣����мȺ����ǻ����ֺ���ȩ����ͬ���칹���� �֡�
��5����֪����SH�������룭OH���ơ�
����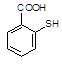 һ���������γɾۺ���Ľṹ��ʽΪ ��
һ���������γɾۺ���Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�춡�ᱽ�Ҵס������ᱽ�Ҵ��dz��������ϣ���Aͨ������ת���ɵõ������л����֪A����Է�������Ϊ104����ȫȼ��ʱ����CO2��H2O�����ʵ���֮��Ϊ2��1����
�ش��������⣺
��1��A�Ľṹ��ʽΪ ��
��2��B��A�ķ�Ӧ������ ��
��3��D��C��Ӧ�����춡�ᱽ�����Ļ�ѧ����ʽΪ ��
��4�����й����춡�ᱽ�����ͱ����ᱽ������˵���У�����ȷ���� ��
a��һ���������ܷ���ˮ�ⷴӦ
b��һ���������ܷ����ӳɷ�Ӧ
c�������ʵ����������л�����ȫȼ�����ĵ�������O2
��5��F�DZ����ᱽ������ͬ���칹�壬F����������F�Ķ��������϶�ֻ��һ��֧����F�Ľṹ��ʽΪ��д�����е�����һ�֣� ��
��6��һ�������£��ɱ���������뱽�Ҵ���ӦҲ�����ɱ����ᱽ������д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�����Ը߷��ӣ��ṹ����ͼ��ʾ��
���������գ�
��1���ø߷�����______�ֵ��壨�ۺϳɸ߷��ӵļ�С���ӣ��ۺ϶��ɡ���Щ���庬�еĹ�
����������___________________��
��2��д������������ʽ����С��ʽ�����ķ��Ӽ䷢�����۷�Ӧ�Ļ�ѧ����ʽ
__________________________________________________��
��3������������ʽ����С�ķ�����һ����������ȫ��ȥ���������ʵĽṹ��ʽΪ
�������ʼӾ����ɵĸ߷��ӣ���ܡ����ܡ���________�����CCl4��Һ
�����ӳɷ�Ӧ�������������Ӿۺϳɻ����������ʵ�������_______��
��4���������������л�Ϊͬϵ����ǣ�д�����п��ܣ��ýṹ��ʽ������
________________________��________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������͡����Ʒ���С����ǽ⡱����һ�ֿڷ�������ҩ��������������֢��ǿ��֢����ʳ֢����ϳ�·�����£�
��1����Ӧ�ڡ��������ڻ�ԭ��Ӧ���� ������ţ���
��2��������F�к� ������̼ԭ�ӣ�������F��G��ˮ���Խ�ǿ���� ��
��3������A��ͬ���칹���У���һ���ܷ���������Ӧ�Һ˴Ź�������ͼ��4�ַ���л� �д������������Һ����������Ӧ�����ӷ���ʽ ��
��4����Ӧ�ٿ���Ϊ������ɣ���1����HCHO�Ⱥ�HN(CH3)2��Ӧ����2���������ٺ�A����ȡ����Ӧ����B����д����1����Ӧ����Ľṹ��ʽ ��
��5����֪�� ��
��
д���� ��HCHO��HN(CH3)2Ϊ�л�ԭ�ϣ��ϳ�
��HCHO��HN(CH3)2Ϊ�л�ԭ�ϣ��ϳ� �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
�ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ҫ���ڷ��ι������ɵȲ�֢����ϳ�·�����£�
˵����D��G��H��ת���зֱ����˼ӳɷ�Ӧ��ˮ�ⷴӦ��
��1��D�к��������ŵ������� ��
��2��C��D�ķ�Ӧ������ ��H�������ҵķ�Ӧ������ ��
��3��E��Fת������һ������HBr�����Լ�X�Ľṹ��ʽ�� ��
��4��H�������� ������̼ԭ�ӡ�
��5��д��ͬʱ��������������A����ͬ���칹��Ľṹ��ʽ ��
���ܷ���������Ӧ������FeCl3������ɫ��Ӧ���۱����ϵ�һ�����ֻ��2�֡�
��6���������ȣ� ���Ǻϳ�ҩƷ����Ҫ�м��塣��д���Ա������ѡ���ȩΪԭ���Ʊ��������ȵĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
���Ǻϳ�ҩƷ����Ҫ�м��塣��д���Ա������ѡ���ȩΪԭ���Ʊ��������ȵĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���A�dz��õ�ʳ���Ϳ�������������ΪC10H12O5���ɷ������±仯��
��֪B����Է�������Ϊ60��������ֻ��һ������C�Ľṹ�ɱ�ʾΪ��
�����С�x��һy��Ϊ�����ţ�����ش��������⣺
��1������ϵͳ��������B������Ϊ ��������һx������Ϊ ���߾���E������Ϊ ��A�Ľṹ��ʽΪ ��
��2����Ӧ�ݵĻ�ѧ����ʽΪ
��3��C�ж���ͬ���칹�壬д������2�ַ�������Ҫ���ͬ���칹��Ľṹ��ʽ �� ��
�ٺ��б��� ���ܷ���������Ӧ �ۺ��з��ǻ�
��4���ӷ��ӽṹ�Ͽ���A���п��������õ���Ҫԭ���� ������ţ���
A�����б��� B�������ʻ� C�����з��ǻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������A����Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ����ͼ��ʾ��
��֪��
a. R�� CH = CHOH���ȶ����ܿ�ת��ΪR�� CH2CHO��
b. R�� CH2CHO���Ժ�����Cu(OH)2��������Һ��������ˮ��R��CH2COOH��Cu2O��
c. H��C��C��H��ԭ����һ��ֱ���ϡ�
����������Ϣ�ش��������⣺
��1��A�ķ���ʽΪ__________________________________________________��
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________________________________��
��3��A�Ľṹ��ʽ��______________________________________________��
��4����Ӧ�ٵĻ�ѧ����ʽ��___________________________________________��
��5��A�ж���ͬ���칹�壬д���ĸ�ͬʱ����(i)�ܷ���ˮ�ⷴӦ(ii)��ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ��______________��____________��________________��________________��
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����±��ṩ�IJ����������Dz���������ѡ������ʵ����Ӧʵ��Ŀ�ĵ���
| ѡ�� | ʵ��Ŀ�� | �������� |
| A | �����Ҵ������������Ļ���� | ��Һ©�����ձ� |
| B | ��pH=1����������100ml, pH=2������ | 100mL����ƿ���ձ�������������ͷ�ι� |
| C | ����ˮ������-KI��Һ�Ƚ�Br2��I2��������ǿ�� | �Թܡ���ͷ�ι� |
| D | ��NH4Cl��Ca��OH��2�����Ʊ����ռ�NH3 | �ƾ��ơ��ձ������ܡ�����ƿ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com