
(1)��ͬ�¶��£������м���4 mol N2��6 mol H2,���ҵ�ѹǿʼ�����ѹǿ��ȣ����з�Ӧ��ƽ��ʱ������NH3�����ʵ���Ϊ________mol(�����и�����ѡ��ֻ���������ͬ)�����ҵ��ݻ�����ݻ�ʼ����ȣ����з�Ӧ��ƽ��ʱ������NH3�����ʵ���Ϊ________mol��
A.С��m B.����m C.��m��2m֮��
D.����2m E.����2m
(2)��ͬ�¶��£������ҵ��ݻ�Ϊ��һ�룬������1 mol NH3��Ҫʹ���з�Ӧ��ƽ��ʱ�������ʵ���������������������д�ƽ��ʱ��ͬ������ʼӦ����______molN2��______mol H2��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꼪��ʡ�Ӽ��и����������¿������ۣ���ѧ���� ���ͣ�ѡ����
�ڼס��������ܱ������з������·�Ӧ��2A��g��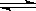 B��g�� + 2C��g�� ��H= m kJ/mol������������ͼ���й�������ȷ����
B��g�� + 2C��g�� ��H= m kJ/mol������������ͼ���й�������ȷ����
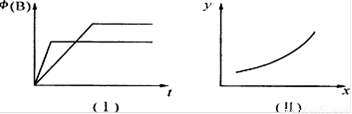
A������Ϊ������ϵ��ͼ�����¶Ȳ�ͬʱ��ʱ����B�����������ϵͼ����m>0
B�����������£�ͼ���ܴ���ѹǿ��ͬʱ��ʱ����B�����������ϵͼ
C�����������£�������������ͼ��ϵ����x����ѹǿʱ��yһ������B���������
D�����������£����ס��������������ͬ����ʼʱ�ֱ�����г���2 mol A�����г���1 molB,3 mol C�����ƽ�����������B���������һ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
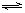 xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��
xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 �¿��� ���ͣ������
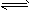 xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2��
xC��g���ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ0.2�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��ƽ��ʱ���ס�����������A�����ʵ�����ȣ���x=____________����ƽ��ʱ���ס�����������A�����ʵ�������ȣ���x=______________��
(2)ƽ��ʱ�ס�����������A��B�����ʵ���֮���Ƿ�(��ǡ���)_______��ȡ�ƽ��ʱ����A���������Ϊ__________��
(3)��ƽ��ʱ�������е�ѹǿ����ȣ�����������ѹǿ֮��Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڼס��������ܱ������з������·�Ӧ��2A��g��![]() B��g�� + 2C��g�� ��H= m kJ/mol������������ͼ���й�������ȷ����
B��g�� + 2C��g�� ��H= m kJ/mol������������ͼ���й�������ȷ����
A������Ϊ������ϵ��ͼ�����¶Ȳ�ͬʱ��ʱ����B�����������ϵͼ����m>0
B�����������£�ͼ���ܴ���ѹǿ��ͬʱ��ʱ����B�����������ϵͼ
C�����������£�������������ͼ��ϵ����x����ѹǿʱ��yһ������B���������
D�����������£����ס��������������ͬ����ʼʱ�ֱ�����г���2 mol A�����г���1 molB,3 mol C�����ƽ�����������B���������һ����ͬ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com