
��16�֣�
��֪CH3COOH��������Ϊ60��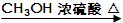 CH3COOCH3��������Ϊ74��
CH3COOCH3��������Ϊ74��
����ֻ��C��H��O�Ļ�����A��F(��ͬ�Ĺ����������ڲ�̼ͬԭ���ϣ�A��һ±����
ֻ��һ��)���й����ǵ�ijЩ��Ϣ����ע��������ķ����ڣ�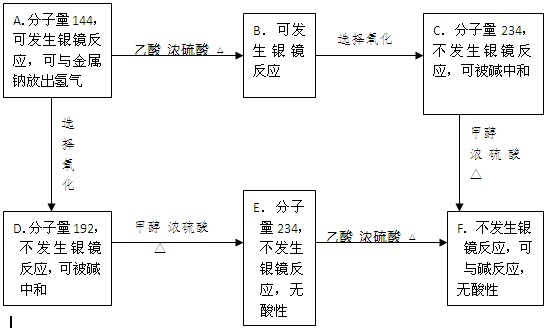
��1���ڻ�����A��F�У������Ľṹ�Ļ������ǣ���д��ĸ���ţ��� ��
��2��A��C�Ľṹ��ʽ��
A ��C ��
��3��д��A�����Ƶ�������ͭ��Һ���ȵĻ�ѧ����ʽ�� ��
��4��д��F�����������������Һ���ȵĻ�ѧ����ʽ�� ��
��5��G��B��ͬ���칹�壬��G������������������
�� G�Ƿ����廯����ֻ��һ�ֹ����š�
��1molGǡ������3mol����������Һ��Ӧ
��G�����в���������ͬһ�ֹ����Ų�������һ��̼ԭ���ϡ�
������������ͬ���칹���У� �֡�����д���֣�
��
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ŨH2SO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ij�¶��£���֪CH3COOH��HClO��H2CO3��H3PO4 ����ƽ�ⳣ�����±���ʾ����0.1mol?L-1�����и���ҺpH�����ǣ������� ������������ĵ���ƽ�ⳣ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
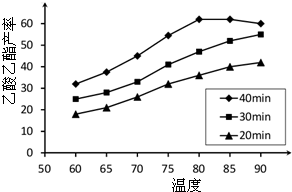
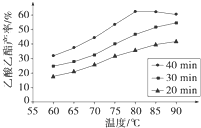 ��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��| ŨH2SO4 |
| �� |
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
| n��CO�� | n��H2O�� | n��H2�� | n��CO2�� | |
| A | 1 | 5 | 2 | 3 |
| B | 2 | 2 | 1 | 1 |
| C | 3 | 3 | 0 | 0 |
| D | 0.5 | 2 | 1 | 1 |
| E | 3 | 1 | 2 | 1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com