
 ��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��| ŨH2SO4 |
| �� |
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
| n��CO�� | n��H2O�� | n��H2�� | n��CO2�� | |
| A | 1 | 5 | 2 | 3 |
| B | 2 | 2 | 1 | 1 |
| C | 3 | 3 | 0 | 0 |
| D | 0.5 | 2 | 1 | 1 |
| E | 3 | 1 | 2 | 1 |
| c(H2)?c(CO2) |
| c(CO)?c(H2O) |
| 3��2 |
| 1��5 |
| 6 |
| 5 |
| c(H2)?c(CO2) |
| c(CO)?c(H2O) |
| 1��1 |
| 2��2 |
| 1 |
| 4 |
| c(H2)?c(CO2) |
| c(CO)?c(H2O) |
| 1��1 |
| 0.5��2 |
| c(H2)?c(CO2) |
| c(CO)?c(H2O) |
| 2��1 |
| 3��1 |
| 2 |
| 3 |
| c2(CO) |
| c(CO2) |
| c(H2)?c(CO) |
| c(H2O) |
| c(H2)?c(CO2) |
| c(CO)?c(H2O) |
| K1 |
| K2 |
| c2(CO) |
| c(CO2) |
| K1 |
| K2 |
| 2842kJ |
| 10mol |

| 3 |
| 5 |
| c(H2)?c(CO2) |
| c(CO)?c(H2O) |
| 4��4 |
| 6��6 |
| 4 |
| 9 |
| 4 |
| 9 |


 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ŨH2SO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ����һ�С����и�����ѧ����ĩ������ѧ�Ծ����������� ���ͣ�ʵ����
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
CH3COOH(l)��C2H5OH(l) CH3COOC2H5(l)��H2O(l) ��H����8.62kJ��mol��1
CH3COOC2H5(l)��H2O(l) ��H����8.62kJ��mol��1
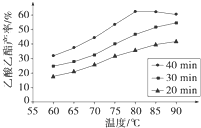
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118�桢78���77�档������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��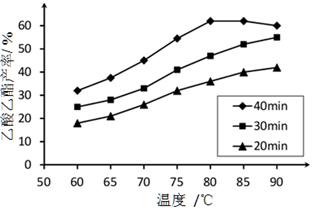
��1�����о�С���ʵ��Ŀ����__________________��
��2��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����___________���ߣ��С�ڡ��������ڡ����ڡ�����
��3����ͼ��ʾ����Ӧʱ��Ϊ40min���¶ȳ���80��ʱ���������������½���ԭ�������______��д����������
��4��ij�¶��£���0.10 mol CH3COOH����ˮ���1 L��Һ��
��ʵ�����ѵ���Ĵ������ռԭ�д������������1.3%������¶���CH3COOH�ĵ���ƽ�ⳣ��K=____________________����ˮ�ĵ�����Բ��ƣ��������Դ������Ũ�ȵ�Ӱ����Բ��ƣ�
�������Һ���ټ���__________mol CH3COONa��ʹ��Һ��pHԼΪ4������Һ����仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�������и���һ�컯ѧ�Ծ��������棩 ���ͣ������
���£��ݻ�Ϊ1
L���������£�����Է�������ת�����䷴Ӧ���̺�������ϵ��ͼ1��ʾ(��֪��2SO2(g)��O2(g) 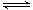 2SO3(g)
��H����196.6 kJ��mol��1)����ش��������⣺
2SO3(g)
��H����196.6 kJ��mol��1)����ش��������⣺
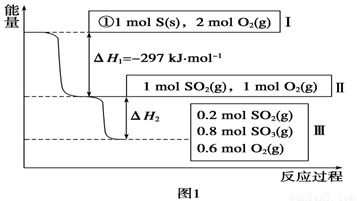
(1)д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ��______________________��
(2)��H2��__________kJ��mol��1��
��.��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
CH3COOH(l)��C2H5OH(l)
 CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118 �桢78 ���77 �档������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��
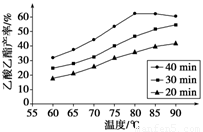
(1)���о�С���ʵ��Ŀ����___________________________________��
(2)60 ���·�Ӧ40 min��70 ���·�Ӧ20 min��ȣ�ǰ�ߵ�ƽ����Ӧ����________����(�С�ڡ��������ڡ����ڡ�)��
(3)��ͼ��ʾ����Ӧʱ��Ϊ40 min���¶ȳ���80 ��ʱ���������������½���ԭ�������_________________________________(д������)��
��.ú�����г����о���ͬ�¶���ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⡣
��֪��CO(g)��H2O(g)  H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
|
�¶�/�� |
400 |
500 |
800 |
|
ƽ�ⳣ��K |
9.94 |
9 |
1 |
�Իش��������⣺
(1)��800 �淢��������Ӧ���Ա��е����ʵ���Ͷ����ݷ�Ӧ��������������Ӧ�����ƶ�����________(ѡ�A��B��C��D��E��)��
n(CO) n(H2O) n(H2) n(CO2)
A 1 5 2 3
B 2 2 1 1
C 3 3 0 0
D 0.5 2 1 1
E 3 1 2 1
(2)��֪��һ���¶��£�C(s)��CO2(g)  2CO(g)ƽ�ⳣ��ΪK��
2CO(g)ƽ�ⳣ��ΪK��
��C(s)��H2O(g) 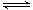 CO(g)��H2(g) ƽ�ⳣ��ΪK1��
CO(g)��H2(g) ƽ�ⳣ��ΪK1��
��CO(g)��H2O(g) 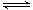 H2(g)��CO2(g) ƽ�ⳣ��ΪK2��
H2(g)��CO2(g) ƽ�ⳣ��ΪK2��
��K��K1��K2֮��Ĺ�ϵ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�����и�����ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ʵ����
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
CH3COOH(l)��C2H5OH(l) CH3COOC2H5(l)��H2O(l) ��H����8.62kJ��mol��1
CH3COOC2H5(l)��H2O(l) ��H����8.62kJ��mol��1
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118�桢78���77�档������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��

��1�����о�С���ʵ��Ŀ����__________________��
��2��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����___________���ߣ��С�ڡ��������ڡ����ڡ�����
��3����ͼ��ʾ����Ӧʱ��Ϊ40min���¶ȳ���80��ʱ���������������½���ԭ�������______��д����������
��4��ij�¶��£���0.10 mol CH3COOH����ˮ���1 L��Һ��
��ʵ�����ѵ���Ĵ������ռԭ�д������������1.3%������¶���CH3COOH�ĵ���ƽ�ⳣ��K=____________________����ˮ�ĵ�����Բ��ƣ��������Դ������Ũ�ȵ�Ӱ����Բ��ƣ�
�������Һ���ټ���__________mol CH3COONa��ʹ��Һ��pHԼΪ4������Һ����仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com