
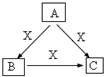
 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�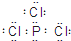 ��
��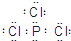 ��
��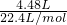 =0.2mol��100mL3mol/L NaOH��ˮ��Һ��n��NaOH��=0.1L��3mol/L=0.3mol��n��CO2����n��NaOH��=0.2mol��0.3mol=2��3=1��1.5������1��1��1��2֮�䣬�ʷ�Ӧ����̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊamol��bmol�����������غ���2a+b=0.3����̼Ԫ���غ���a+b=0.2���������̣����a=0.1��b=0.1��̼�����̼�����ˮ�⣬��Һ�ʼ���c��OH-����c��H+����̼�����ˮ��̶ȱ�̼������Ĵ�c��HCO3-����c��CO32-����ˮ��̶Ⱥ�С��c��CO32-��ԭ����c��OH-������Һ���������Ѷ���ʣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
=0.2mol��100mL3mol/L NaOH��ˮ��Һ��n��NaOH��=0.1L��3mol/L=0.3mol��n��CO2����n��NaOH��=0.2mol��0.3mol=2��3=1��1.5������1��1��1��2֮�䣬�ʷ�Ӧ����̼������̼�����ƣ���̼������̼�����Ƶ����ʵ����ֱ�Ϊamol��bmol�����������غ���2a+b=0.3����̼Ԫ���غ���a+b=0.2���������̣����a=0.1��b=0.1��̼�����̼�����ˮ�⣬��Һ�ʼ���c��OH-����c��H+����̼�����ˮ��̶ȱ�̼������Ĵ�c��HCO3-����c��CO32-����ˮ��̶Ⱥ�С��c��CO32-��ԭ����c��OH-������Һ���������Ѷ���ʣ�c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
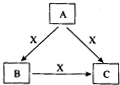 A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
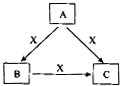 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com