

���� ����ɰ����Ҫ�ɷ�Na2B4O7•10H2O��Ϊԭ�������������ƾ��壬��ɰ�м�������������Һ������ҺPH=10-11�����˵õ�����A��NaBO2����Һ���������������˵õ������ƾ��壬
��1��������֪��Na2B4O7��NaOH��Ӧ��NaBO2�����ԭ���غ�͵���غ㼴��д�����ӷ���ʽ��
��2�������ܶȻ����������������Ũ�ȣ��ٸ���ˮ�����ӻ���������������Ũ�ȣ�����pH��
��3�����������Ŀ����У����ȽϸߵĹ��������ǽ��ȶ��ģ���40���ʪ�Ŀ����зֽ⣬���ų���������63��ʱ���������Ľᾧˮ���ֽ⣬�������Թ̿飬����70��ʱʧȥ3���ᾧˮ���γ�һˮ�һˮ����������Ϊһ�ָ�Ч�ȶ�����ϵƯ����
��4������������60�����ϵ���ˮ�в��ܳ���ܽⲢ�������ã���̼���ƽϹ��������ڵ������ܽ����ܺã�
��� �⣺��1��������֪��д����Ӧ��Ͳ��������
Na2B4O7+NaOH��NaBO2+�������������غ��֪δ֪��ΪH2O��
��ƽ��ʽΪ��Na2B4O7+2NaOH�T4NaBO2+H2O�����ӷ���ʽΪ��B4O72-+2OH-=4BO2-+H2O��
�ʴ�Ϊ��B4O72-+2OH-=4BO2-+H2O��
��2��Mg2+������PH�������£�Ksp�Tc��Mg2+����c��OH-��2 ����c��OH-��Ũ��Ϊx������Ksp����ʽ�ã�
0.056��x2�T5.6��10-12��
���x�T1.0��10-5��
C��H+��=$\frac{1��1{0}^{-14}}{1��1{0}^{-5}}$�T1��10-9��
pH�T9���ʵ�����Һ��pH=9ʱ���ſ�ʼ���ֳ�����
�ʴ�Ϊ��9��
��3�����������Ŀ����У����ȽϸߵĹ��������ǽ��ȶ��ģ���40���ʪ�Ŀ����зֽ⣬���ų���������63��ʱ���������Ľᾧˮ���ֽ⣬�������Թ̿飬����70��ʱʧȥ3���ᾧˮ���γ�һˮ���Ӧ�Ļ�ѧ����ʽΪ��NaBO3•4H2O$\frac{\underline{\;����70��\;}}{\;}$NaBO3•H2O+3H2O��һˮ�������Ǵ���ˮ����Ĺ����������ѵ�����ˮ���Ӻ�õ���Ʒ������ˮ����������ȣ�һˮ�������ƾ��и��ߵĻ��������������ߵ����ȶ��Ժ��ߵ��ܽ����ʣ�
�ʴ�Ϊ��NaBO3•4H2O$\frac{\underline{\;����70��\;}}{\;}$NaBO3•H2O+3H2O��B��
��4������������60�����ϵ���ˮ�в��ܳ���ܽⲢ�������ã���̼���ƽϹ��������ڵ������ܽ����ܺã���ˮ��Һ�ʼ��ԣ����Էֽ�Ϊ̼���ƺ������⣬�и��õ�Ư��Ч�����ʴ�Ϊ����̼���ƽϹ��������ڵ������ܽ����ܺã��۸�ߣ�
���� ���⿼��������ѡ������жϣ��������ѵ㣬����ʱҪ���������Ŀ����������ϵ�ͱ������ݣ�����������ϵ�����������ĸ�����ٽ��⣮


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ�ӵĽṹʾ��ͼ�� | |
| B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | |
| C�� | ���������ӵĵ���ʽ�� | |
| D�� | ������Ϊ146��������Ϊ92 ���ˣ�U��ԭ�ӣ�${\;}_{92}^{146}$U |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ʯīת��Ϊ���ʯ�����ȷ�Ӧ | |
| B�� | CO��g��+H2O��g���TCO2��g��+H2��g�������ȷ�Ӧ | |
| C�� | ��ͬ�����£�������S��g����S��s���������Ƚϣ�S��s���ϴ� | |
| D�� | ���ױȺ����ȶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg2+��OH-��NH4+��Na+ | |
| B�� | c��H+��ˮ=1.0��10-12mol•L-1����Һ�У�S2-��Na+��OH-��K+ | |
| C�� | S2-��Cl-��Al3+��SO42- | |
| D�� | S2-��K+��Fe3+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
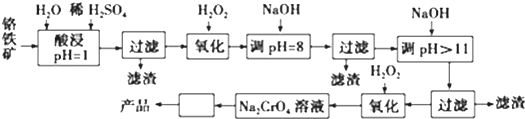
| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cr3+ |
| ��ʼ����ʱ��pH | 2.7 | 7.6 | 9.0 | -- | -- |
| ������ȫʱ��pH | 3.7 | 9.6 | 11.0 | 8 | 9����9�ܽ⣩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | NaOH/mol•L-1 | HA/mol•L-1 | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | C | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1molFeI2������������Ӧʱת�Ƶĵ�����Ϊ3NA | |
| B�� | 1L2mol•L-1 K2S��Һ��S2-��HS-������Ϊ2NA | |
| C�� | ��״���£�22.4L��CCl4�к��е�CCl4������ΪNA | |
| D�� | 50mL18mol•L-1Ũ����������ͭ�ȷ�Ӧ��ת�Ƶĵ�����Ϊ1.8NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �٢� | B�� | �ۢ� | C�� | �ڢ� | D�� | �٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼��ʯ��ˮ��Ӧ��CO2+2OH-�TCO32-+H2O | |
| B�� | ��ˮ��ͨ��������SO2��Br2+SO2+2H2O�T2Br-+SO42-+4H+ | |
| C�� | Cu����ϡ���Cu++2H++NO3-�TCu2++NO2��+H2O | |
| D�� | Na2O2����ˮ����O2��Na2O2+H2O�T2Na++2OH-+O2�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com