
ij�������Թ�ҵ��ˮ�к���һ������Fe3+��Cu2+��Au3+�����ӡ����������ͼ�еĹ������̣����ó��õ��ᡢ���ҵ�����еķ���м���ӷ�ˮ�л��ս𣬲�����һ���������������ͭ��




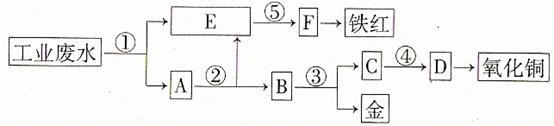
 ��д����հס�
��д����հס� ��1��ͼ�б�Ŵ���������Ӧ���ʷֱ��Ǣ���������������������������������������������������������������������
��1��ͼ�б�Ŵ���������Ӧ���ʷֱ��Ǣ��������������������������������������������������������������������� ��2��д���ٴ�������Ӧ�����ӷ���ʽ������������������������������������������д���۴�������Ӧ�Ļ�ѧ����ʽ����������������������������������������������������
��2��д���ٴ�������Ӧ�����ӷ���ʽ������������������������������������������д���۴�������Ӧ�Ļ�ѧ����ʽ���������������������������������������������������� ��3������Ļ�ѧʽΪ���������������������ֱ�д�����������ͭ�ڹ�ҵ�ϵ�һ����Ҫ��;������������������������������������������ͭ��������������������������������
��3������Ļ�ѧʽΪ���������������������ֱ�д�����������ͭ�ڹ�ҵ�ϵ�һ����Ҫ��;������������������������������������������ͭ��������������������������������
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �������� | Ksp | pH��10-1 mol?L-1�� | pH��10-5 mol?L-1�� |
| Fe3+ | 4.0��10-38 | 2.7 | 3.7 |
| Cr3+ | 6.0��10-31 | 4.3 | 5.6 |
| Cu2+ | 2.2��10-20 | 4.7 | 6.7 |
| Ca2+ | 4.0��10-5 | 12.3 | 14.3 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�Ͼ�ѧ�����ר��ѧУ����9���²⻯ѧ�Ծ����������� ���ͣ������
��10�֣����Ź�ҵ��Ѹ�ٷ�չ�������ķ�ˮ��ˮ�����ȾҲ�������ء�ͨ��������Һ��pH�Թ�ҵ��ˮ�еĽ������ӽ��з�����ʵ�ʹ����о���ʹ�õķ������±��dz����½������������Ksp(�����ܽ�ƽ�ⳣ��)�ͽ���������ijŨ���¿�ʼ���������pH(����Ũ��Ϊ��ӦpHʱ��Һ���йؽ������Ӳ�����������СŨ�ȣ�����Һ�н�������Ũ��С��10-5 mol?L-1ʱͨ����Ϊ�����ӳ�����ȫ)��
| �������� | Ksp | pH(10-1 mol?L-1) | pH(10-5 mol?L-1) |
| Fe3+ | 4.0��10-38 | 2.7 | 3.7 |
| Cr3+ | 6.0��10-31 | 4.3 | 5.6 |
| Cu2+ | 2.2��10-20 | 4.7 | 6.7 |
| Ca2+ | 4.0��10-5 | 12.3 | 14.3 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ����9���²⻯ѧ�Ծ��������棩 ���ͣ������
��10�֣�������ҵ��Ѹ����չ����������ˮ��ˮ�����ȾҲ�������ء�ͨ��������Һ��pH�Թ�ҵ��ˮ�еĽ������ӽ��з�����ʵ�ʹ����о���ʹ�õķ������±��dz����½������������Ksp(�����ܽ�ƽ�ⳣ��)�ͽ���������ijŨ���¿�ʼ���������pH(����Ũ��Ϊ��ӦpHʱ��Һ���йؽ������Ӳ�����������СŨ�ȣ�����Һ�н�������Ũ��С��10-5 mol•L-1ʱͨ����Ϊ�����ӳ�����ȫ)��
|
�������� |
Ksp |
pH(10-1 mol•L-1) |
pH(10-5 mol•L-1) |
|
Fe3+ |
4.0��10-38 |
2.7 |
3.7 |
|
Cr3+ |
6.0��10-31 |
4.3 |
5.6 |
|
Cu2+ |
2.2��10-20 |
4.7 |
6.7 |
|
Ca2+ |
4.0��10-5 |
12.3 |
14.3 |
(1)ij���ų��ķ�ˮ�к���Cu2+��Fe3+�������Ũ�Ⱦ�С��0.1 mol•L-1��Ϊ��ȥ���е�Fe3+������ͭ������Ƶ�pH��Χ��_______________________________��
(2)Ϊ�˴�������Cr2O72-������Һ�Ĺ�ҵ��ˮ���������·��������ˮ�м�������NaCl����FeΪ�缫���е�⣬����һ��ʱ�䣬��Cr(OH)3��Fe(OH)3���������ų����Ӷ�ʹ��ˮ�и����������ŷű���
��Cr2O72-ת��ΪCr3+�����ӷ���ʽΪ______________________��
��pH�Է�ˮ��Cr2O72-ȥ���ʵ�Ӱ����ͼ������Ϊ����������Һ��pHȡֵ��______��Χ�ڶԽ��ͷ�ˮ�еĸ���������������˵�����ɣ�_________________________________��
[ע��ȥ����(��)=[(c0-c)��co]��100����ʽ�У�co������ǰ��ˮ��Cr2O72-Ũ�ȣ�c���������ˮ��Cr2O72-��Ũ��]

(3)����ת����������Ҳ����ҪӦ�á����磬��Na2CO3��Һ���Խ���¯ˮ���е�CaSO4ת��Ϊ�����ɶ��������CaCO3���ó���ת���ﵽƽ��ʱ����ƽ�ⳣ��K��_________(д��ֵ)��[��֪Ksp (CaSO4)=9.1x10-6��Ksp (CaCO3)=2.8��10-9]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(10��)
���Ź�ҵ��Ѹ�ٷ�չ�������ķ�ˮ��ˮ�����ȾҲ�������ء�ͨ��������Һ��pH�Թ�ҵ��ˮ�еĽ������ӽ��з�����ʵ�ʹ����о���ʹ�õķ������±��dz����½������������Ksp(�����ܽ�ƽ�ⳣ��)�ͽ���������ijŨ���¿�ʼ���������pH(����Ũ��Ϊ��ӦpHʱ��Һ���йؽ������Ӳ�����������СŨ�ȣ�����Һ�н�������Ũ��С��10-5 mol��L-1ʱͨ����Ϊ�����ӳ�����ȫ)��
| �������� | Ksp | pH(10-1 mol��L-1) | pH(10-5 mol��L-1) |
| Fe3+ | 4.0��10-38 | 2.7 | 3.7 |
| Cr3+ | 6.0��10-31 | 4.3 | 5.6 |
| Cu2+ | 2.2��10-20 | 4.7 | 6.7 |
| Ca2+ | 4.0��10-5 | 12.3 | 14.3 |
(1)ij���ų��ķ�ˮ�к���Cu2+��Fe3+�������Ũ�Ⱦ�С��0.1 mol��L-1��Ϊ��ȥ���е�Fe3+������ͭ������Ƶ�pH��Χ��_______________________________��
(2)Ϊ�˴�������Cr2O72-������Һ�Ĺ�ҵ��ˮ���������·��������ˮ�м�������NaCl����FeΪ�缫���е�⣬����һ��ʱ�䣬��Cr(OH)3��Fe(OH)3���������ų����Ӷ�ʹ��ˮ�и����������ŷű���
 ��Cr2O72-ת��ΪCr3+�����ӷ���ʽΪ______________________��
��Cr2O72-ת��ΪCr3+�����ӷ���ʽΪ______________________��
��pH�Է�ˮ��Cr2O72-ȥ���ʵ�Ӱ������ͼ������Ϊ����������Һ��pHȡֵ��______��Χ�ڶԽ��ͷ�ˮ�еĸ���������������˵�����ɣ�____________________________________________��
[ע��ȥ����(��)=[(c0-c)��co]��100����ʽ�У�co����ǰ��ˮ��Cr2O72-��Ũ�ȣ�c���������ˮ��Cr2O72-��Ũ��]
(3)����ת����������Ҳ����ҪӦ�á����磬��Na2CO3��Һ���Խ���¯ˮ���е�CaSO4ת��Ϊ�����ɶ��������CaCO3���ó���ת���ﵽƽ��ʱ����ƽ�ⳣ��K��_________(д��ֵ)��[��֪Ksp (CaSO4)=9.1x10-6��Ksp (CaCO3)=2.8��10-9]
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com