
�����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ��������ʵ����Ҳ����������ͼ��ʾ��װ����ȡ������������ش��������⡣

(1)�Ҵ������й����ŵ�������________________��
(2)�Թ�a�м��뼸�����Ƭ��Ŀ����_________��
(3)�Թ�a�з�����Ӧ�Ļ�ѧ����ʽΪ_____________________________����Ӧ������____________________��
(4)��Ӧ��ʼǰ���Թ�b��ʢ�ŵ���Һ��____________��
(5)����____________�ķ������Ƶõ������������������
(1)�ǻ� (2)��ֹ����
(3)CH3COOH��C2H5OH
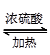 CH3COOC2H5��H2O
����������Ӧ
CH3COOC2H5��H2O
����������Ӧ
(4)����̼������Һ (5)��Һ
����������1���Ҵ����ڴ��࣬�����ǻ���
��2��������ӦС�ڼ��ȣ�����Ϊ��ֹ��Һ����ʱ���У���Ҫ�������Ƭ��
��3��������Ҵ�����������Ӧ�ķ���ʽΪ
CH3COOH��C2H5OH
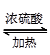 CH3COOC2H5��H2O��
CH3COOC2H5��H2O��
��4���������������к���������Ҵ������ʣ�������Ҫ���뱥�͵�̼������Һ���ӡ�
��5����������������ˮ����Һ���ɡ�


 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ��������ʵ����Ҳ������������ͼ��ʾ��װ����ȡ������������ش��������⣮
�����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ��������ʵ����Ҳ������������ͼ��ʾ��װ����ȡ������������ش��������⣮ CH3COOC2H5+H2O
CH3COOC2H5+H2O CH3COOC2H5+H2O
CH3COOC2H5+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�����Ϊ�ڴ������������������ζ��������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�����Ϊ�ڴ������������������ζ��������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ��������ʵ����Ҳ��������ͼ��ʾ��װ����ȡ������������ش��������⣮
�����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ��������ʵ����Ҳ��������ͼ��ʾ��װ����ȡ������������ش��������⣮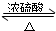 CH3COOC2H5+H2O
CH3COOC2H5+H2O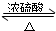 CH3COOC2H5+H2O
CH3COOC2H5+H2O ״Һ�������������B�е�Һ��������Ҫ�õ�����Ҫ����������
״Һ�������������B�е�Һ��������Ҫ�õ�����Ҫ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com