
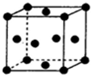
 A��B��C��D��E��F��ԭ���������ε�����ǰ������Ԫ�أ�����C��Eͬ���壬��E��ԭ��������C��������A�ֱ���B��C�����γ�10���ӷ��ӣ�B��C������������֮��2��3��Fԭ�ӵ�������������A��ͬ��������������������������D2C2��ˮ��Ӧ����C�ĵ��ʣ�����Һ��ʹ��̪��Һ�ȱ�����ɫ���ݴ˻ش��������⣺
A��B��C��D��E��F��ԭ���������ε�����ǰ������Ԫ�أ�����C��Eͬ���壬��E��ԭ��������C��������A�ֱ���B��C�����γ�10���ӷ��ӣ�B��C������������֮��2��3��Fԭ�ӵ�������������A��ͬ��������������������������D2C2��ˮ��Ӧ����C�ĵ��ʣ�����Һ��ʹ��̪��Һ�ȱ�����ɫ���ݴ˻ش��������⣺| 1 |
| 8 |
| 1 |
| 2 |
| 6+2 |
| 2 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Cu2++2OH-=Cu��OH��2�� CuSO4+Ba��OH��2=Cu��OH��2��+BaSO4�� |
| B��CO32-+2H+=CO2��+H2O NaHCO3+HCl=NaCl+CO2��+H2O |
| C��H++OH-=H2O NaOH+NH3?H2O=NH4Cl+H2O |
| D��SO42-+Ba2+=BaSO4�� BaCl2+H2SO4=BaSO4��+2HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ��Ӧԭ������������������Ź㷺��Ӧ�ã����á���ѧ����ת�Ʒ����Ʊ�TaS2��
��ѧ��Ӧԭ������������������Ź㷺��Ӧ�ã����á���ѧ����ת�Ʒ����Ʊ�TaS2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
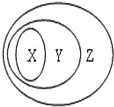 ����ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�в���ȷ���ǣ�������
����ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�в���ȷ���ǣ�������| ѡ�� | X | Y | Z |
| A | ������ | ������ | ������ |
| B | ����� | ���ӻ����� | ������ |
| C | ���� | ��ɢϵ | ����� |
| D | ���������� | ���������� | ������ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��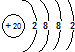 |
B��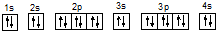 |
| C��1s22s22p63s23p64s2 |
| D��1s22s22p63s23p63d2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
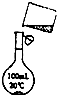 Ϊ��ȥ�����е�Ca2+��Mg2+��Fe3+��SO42-�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�����
Ϊ��ȥ�����е�Ca2+��Mg2+��Fe3+��SO42-�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�����| �ܽ� |
| �� |
| BaCl2 |
| �� |
| NaOH |
| �� |
| Na2CO3 |
| �� |
| ���� |
| �� |
| �������� |
| �� |
| �������ᾧ����� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com