
| c(Pb2+) |
| c(NO3-) |
| 1 |
| 2 |
| 1 |
| 2 |
| 1.0mg/ml��100ml��10-3g/mg |
| 207g/mol |
| 0.490+0.510 |
| 2 |


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| c(NO3-) |
| c(Pb2+) |
| c(NO3-) |
| c(Pb2+) |
| ���� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl- |
| ����ǰŨ��/��mg?L-1�� | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ��/��mg?L-1�� | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 2NH3��g��
2NH3��g��| t/K | 298 | 398 | 498 | �� |
| K/��mol?L-1��2 | 4.1��106 | K1 | K2 | �� |
 [N2H5?H2O]++H+
[N2H5?H2O]++H+ [N2H5?H2O]++H+
[N2H5?H2O]++H+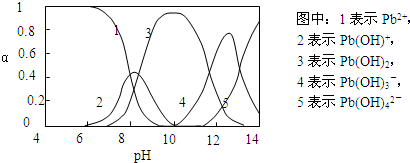
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ������ѧ���������¿������ۣ���ѧ���� ���ͣ������
���ǵ����Ϻ����ḻ��һ��Ԫ �أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش�
�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش� ��1����һ������ĺ����ܱ������У��������»�ѧ��Ӧ�� N2(g)+3H2(g) 2NH3(g)
��1����һ������ĺ����ܱ������У��������»�ѧ��Ӧ�� N2(g)+3H2(g) 2NH3(g)
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
| t/K | 298 | 398 | 498 | �� |
| K/(mol��L��1)2 | 4.1��106 | K1 | K2 | �� |
 �������⣺
�������⣺ �ȵ�����˳����ȷ���� ������ţ���
�ȵ�����˳����ȷ���� ������ţ��� |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ�ij���������У�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ������
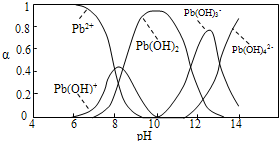
ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2����Pb(OH)����Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ��

(1)Pb(NO3)2��Һ�У� ________2(�>����������<��)��������Һ�е����Ȼ����Һ��
________2(�>����������<��)��������Һ�е����Ȼ����Һ�� ���ӣ����ܵ�ԭ����________________________________��
���ӣ����ܵ�ԭ����________________________________��
(2)��Pb(NO3)2��Һ�е���ϡNaOH��Һ��pH��8ʱ��Һ�д��ڵ�������(Na������)��__________��pH��9ʱ��Ҫ��Ӧ�����ӷ���ʽΪ_______________________��
(3)ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������±���
|
���� |
Pb2�� |
Ca2�� |
Fe3�� |
Mn2�� |
Cl�� |
|
����ǰŨ��/(mg��L��1) |
0.100 |
29.8 |
0.120 |
0.087 |
51.9 |
|
������Ũ��/(mg��L��1) |
0.004 |
22.6 |
0.040 |
0.053 |
49.8 |
�ϱ��г�Pb2���⣬����Ǧ�����������ӵ�ȥ��Ч����õ���________��
(4)��� ����Ǧ��(��EH��ʾ)��Ǧ��Ҫ�����ķ�Ӧ������Ϊ��2EH(s)��Pb2�� E2Pb(s)��2H������Ǧ�������pH��ΧΪ(
)
E2Pb(s)��2H������Ǧ�������pH��ΧΪ(
)
A��4��5 B��6��7 C��9��10 D��11��12
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com