




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ��һ��ѧ����ĩ������ѧ�Ծ� ���ͣ�ʵ����
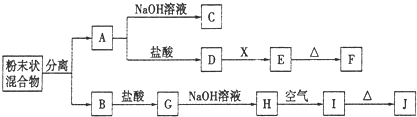
��12��ij��ѧ��ȤС���ú�A��B���ֽ������ʵķ�ĩ״������������ʵ�飬��ת����ϵ��ͼ��ʾ(���ַ�Ӧ���������δ�г�)������EΪ��ɫ������IΪ���ɫ������
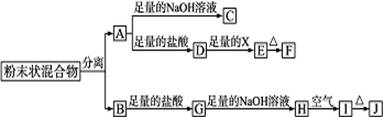
(1)д���������ʵĻ�ѧʽ��F____________________��G_______________
(2)�����ĩ״�������ķ�����__________________________________
(3)D��Eת���У�����������X���Լ�X������________
A������NaCl��Һ B��NaOH��Һ C����ˮ D��Ba(OH)2��Һ
(4)д������ת���Ļ�ѧ����ʽ��
A��C��_____________________________________________________________________
H��I��____________________________________________________________________
��5��������B����ϡ�����еĻ�ѧ��Ӧ����ʽΪ��____________________________________
��6������G��Һ�е������ӽ��м��飺�������Լ���KSCN��Һ����ˮ����Ƽ���G��Һ�������ӵķ�����
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com