
��16�֣�
��1����ͼΪʵ�����Ʊ����ռ�����HCl��װ�á�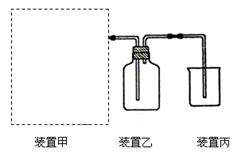
���Ʊ�HCl����ҩƷΪŨ�����Ũ���ᣬ������װ��Ӧѡ����ͼ�е� ��
�� ���������Ũ�����Ũ�����Ʊ�HCl�����ԭ�� ��
�� װ�ñ��������չ�����HCl���壬Ϊ��ֹ���������ձ���Ӧ��װ��ˮ�� ��
��2������Ϊ����ȡ����Ӧ��̽��ʵ�顣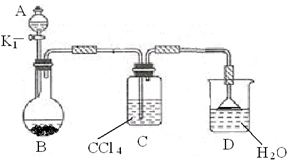
�� ����ͼ��ʾ��װ��ͼ���Ӻø�������
�� ����װ�õ������ԡ�
�� ��A�м��������ı���Һ��Ļ��Һ�壬��B�м����������ۣ�������Ƥ������K1����ʼ���з�Ӧ�������������Һ�ر�K1��
д��B�з�����Ӧ�Ļ�ѧ����ʽ ��
װ��C�������� ��
�� ��Ӧ���������Թ�ȡ����D�е���Һ������
�������Լ���������֤��B�е�ȡ����Ӧ�Ѿ�������
�� ��B�еĹ�Һ���������ˣ������Һ���������ͼ�������ƺ��б�������屽��
�����Լ�Ϊ ����������Ϊ ��
��16�֣�ÿ��2�֣�
��1���� c
�� Ũ�������ǿ��ˮ�ԣ�������Ũ�����е�ˮ��ͬʱ�ų��������ȣ��ٽ���HCl����Ļӷ������Կ�����Ũ�����Ũ�����Ʊ�HCl����
�� CCl4
��2����
������ͱ���������
�� ����AgNO3��Һ������е���ɫ�������ɡ�
�� NaOH��Һ������Na2SO3��Һ�Ⱥ����𰸣� �� ��Һ������
���������������1��������Ũ������ˮ���ʱ���ȼ�Ũ����Ļӷ�����ȡ�����Ȼ��⣬������ȣ�����ѡ��cװ�ã�
��������Ũ�����Ũ��������ԣ�Ũ�������ǿ��ˮ�ԣ�������Ũ�����е�ˮ��ͬʱ�ų��������ȣ��ٽ���HCl����Ļӷ������Կ�����Ũ�����Ũ�����Ʊ�HCl���壻
��Ϊ��ֹ������������ˮ�⣬��Ӧ�����ˮ�ܶȴ����Ȼ��ⲻ�������е�Һ�����ʣ�����ѡ��CCl4;
��2����B�з����������ڴ����������������屽�ķ�Ӧ����ѧ����ʽΪ
���뱽���ӷ���C��ʢ�����Ȼ�̼���������������ջӷ�����ͱ���������
�ܻӷ����屻C���գ�����������ȡ����Ӧ�������������廯�⣬�廯�ⲻ�������Ȼ�̼����ˮ��������������ȡ����Ӧ����D�����廯����ڣ���������AgNO3��Һ�����е���ɫ�������ɡ�
�ݴ��屽�к����塢�����ʣ�����Ӧ��ȥ����������������Һ�ɳ�ȥ�壬��ʱ��Һ�ֲ㣬��Һ����Ͳ��к������屽��������ɵ��屽��
���㣺����Ũ�������ˮ�ԡ�Ũ����Ļӷ��Ե�Ӧ�ã��屽����ȡ����ѧ����ʽ����д�����ʵij���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���п�״�����ڳ�������ȫ������������Ũ�������
| A��Au | B��Cu | C��Fe | D��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���г�����ȷ�����������ϵ���ǣ� ��
| ѡ�� | ������ | ������ |
| A | SO2��Ư���� | SO2��ʹ��ˮ��ɫ |
| B | SiO2�е����� | SiO2�������Ʊ����ά |
| C | Ũ������ǿ������ | Ũ��������ڸ���H2��CO |
| D | Fe3+��ǿ������ | FeCl3��Һ�����ڻ��շϾɵ�·���е�ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣���A��D��E��G��������ɵĸ���W�Ļ�ѧʽΪxAaDd��yE2D��zG��4.704gW����ˮ��һ�������������Һ��������������Ϊ3.408g��ͬ������W��5.13g����������ֻ��Ⱥ���ȣ����ɵ�����������������Һ������յ�Al(OH)3 0.624g�����Ⱥ�IJ�����ˮ����ܽ����ˣ���Һ�Է�̪Ϊָʾ����0.400mol/L H2SO4�ζ����յ㣬��ȥH2SO4 15.0mL���ζ���Ϻ���Һ�м�ⲻ���κν������Ӻ�������ӣ�����������ϴ��ʱ�����ܽ⣬��һ��ʯ̿������ɫ�Ļ�ɫ��Һ�����������ͼ����жϣ�
��1�������D��E��G����������________��_______��_________��
��2��a��d�ı�ֵΪ_________��
��3�����εĻ�ѧʽΪ________����д�����㲽�裩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�Ϊ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС����̼�ظ�(������̼�ĺϽ�)����������̽�����
[̽��һ]��1������ȥ�����������������(̼�ظ�)������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ���� ��
��2����ȡ̼�ظ�6��0 g����15��0 mLŨ�����У����ȣ���ַ�Ӧ��õ���ҺX���ռ����������Y��
�ټ�ͬѧ��ΪX�г�Fe3��֮����ܺ���Fe2������Ҫȷ�����е�Fe2����Ӧѡ�� (ѡ�����)��
A��KSCN��Һ����ˮ B�����ۺ�KSCN��Һ
C��Ũ��ˮ D������KMnO4��Һ
����ͬѧȡ560 mL(��״��)����Yͨ��������ˮ�У�Ȼ���������BaCl2��Һ�����ʵ�������ø������4��66 g���ɴ���֪����Y��SO2���������Ϊ ��
[̽����]��������ʵ����SO2��������ķ�������ͬѧ��Ϊ����Y�л����ܺ���Q1��Q2�������壬����Q1���壬�ڱ�״���£��ܶ�Ϊ0��0893 g��L��1��Ϊ�����������̽��ʵ��װ��(�����й�������ȫ��Ӧ)��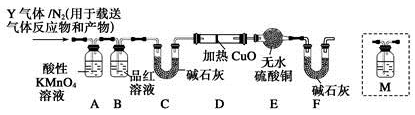
��3��װ��B���Լ��������� ��
��4������Y�����е�Q2������������ɵ� (�û�ѧ����ʽ��ʾ)��
��5����֪ϴ��ƿM��ʢװ����ʯ��ˮ��Ϊȷ��Q2�Ĵ��ڣ�����װ��������ϴ��ƿM�� (�����)��
A��A֮ǰ B��A��B��
C��B��C�� D��C��D��
��6���������Y�к���Q1��Ԥ��ʵ������Ӧ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ����֤ľ̿�ɱ�ŨH2SO4������CO2��ѡ����ͼ��ʾ����(�ں�����)��װ��ʵ��װ�ã�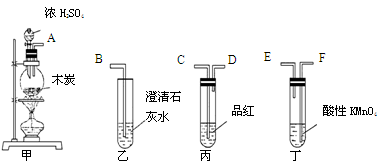
��1���簴������������������������װ�õ���ȷ˳����(����ӿ���ĸ)��
�� �� �� �� �� ��
��2�������ҡ���Ӧ��������ʵ������ű����Ѽ����CO2?
���� ____ ������ ______ ��
��3����������KMnO4��Һ�������� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�������������Ҫ�Ĺ�ҵԭ�ϣ�̽�����Ʊ����������ʾ��зdz���Ҫ�����塣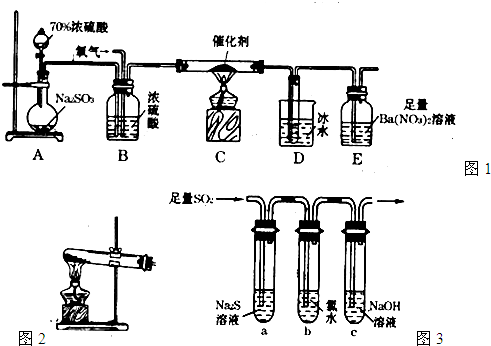
��1��ʵ������ͼ1װ�òⶨSO2������ΪSO3��ת���ʡ�����֪SO3�۵�Ϊ16��8�棬�����������װ��ʱ�ֱ���ȫ���գ��Һ��Կ�����CO2��Ӱ�졣��
�ټ���ʹ�÷�Һ©����Բ����ƿ�еμ�Ũ����IJ����� ��
��ʵ������У���Ҫͨ����������д��һ������ͼ2��ʾװ����ȡ�����Ļ�ѧ����ʽ�� ��
�۵�ֹͣͨ��SO2Ϩ��ƾ��ƺ���Ҫ����ͨһ��ʱ�����������Ŀ���ǣ� ��
��ʵ���������װ��D���ӵ�����Ϊmg��װ��E�в�����ɫ����������Ϊng�����
�����¶��������ת������ ���ú���ĸ�Ĵ���ʽ��ʾ�����û���
��2��ijѧϰС���������ͼ3װ����֤��������Ļ�ѧ���ʡ�
����˵������������������Ե�ʵ������Ϊ�� ��
��Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ�Թ�b�е���Һ�����ݣ��ֱ��������ʵ�顣
����I�����һ����Һ�м���AgNO3��Һ���а�ɫ��
�����ɡ�
����II����ڶ�����Һ�м���Ʒ����Һ����ɫ��ȥ��
����III�����������Һ�м���BaC12��Һ��������ɫ����
��������������� ���I������II������III������
�Թ�b������Ӧ�����ӷ���ʽ�� ��
�۵�ͨ������������Թ�c����Һ������ʱ������Һ��
c(Na+)= ���ú�����Ũ�ȵĴ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���͡�������š��ɴ��Ļ��ȼ�ϳ�Һ̬˫��ˮ�⣬������һ��Һ̬���⻯�����֪�û���������Ԫ�ص���������Ϊ12�� 5%����Է�������Ϊ32���ṹ�������ָ÷��ӽṹ��ֻ�е�����
��1���õ��⻯����ĵ���Ϊ ��
��2������������Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��NH3�����е�Nԭ����һ�Թ¶Ե��ӣ��ܷ�����Ӧ��NH3+HCl=NH4Cl����д���������⻯����ͨ����������ʱ��������Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�赥�ʼ��仯����Ӧ�úܹ㡣��ش��������⣺
(1)�Ʊ���뵼����ϱ����ȵõ��ߴ��衣���ȼ���(SiHCl3)��ԭ���ǵ�ǰ�Ʊ��ߴ������Ҫ��������������ʾ��ͼ���£�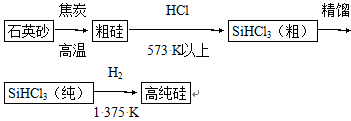
��д���ɴ�SiHCl3�Ʊ��ߴ���Ļ�ѧ��Ӧ����ʽ____________________��
�������Ʊ����̱����ϸ������ˮ��������SiHCl3��ˮ���ҷ�Ӧ����H2SiO3��HCl����һ�����ʣ�д����ƽ�Ļ�ѧ��Ӧ����ʽ____________________��H2��ԭSiHCl3������������O2����������ĺ����____________________��
(2)�����йع���ϵ�˵����ȷ���� (����)��
A�����ʹ軯ѧ�����ȶ��������Ա�ǿ����Һ��ʴ
B�����������跴Ӧ���ʲ�������Ϊ��Һ�ⵥ����
C����ͨ�������ɴ��ʯ��ʯ��ʯӢɰ�Ƴɵģ����۵�ܸ�
D�����ά����Ҫ�ɷ���SiO2
(3)������ˮ��Һ�׳�ˮ������ȡ������������Һ���Թ��У���μ������ᣬ��д��ʵ�����������(�û�ѧ����ʽ˵��)_________________________ ____��
(4)�����������ܵ�����ʱ����Ҫʹ��һ���������ǽ���������ֲ�����ڣ����ֲ�����________(����ĸ)��
A�����½ṹ�մ� ��B�������մɡ� C�������մ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com