
����Ŀ���������ѧ֪ʶ�ش��������⣺
�� | ���볣�� |
CH3COOH | K = 1.8��10 -5 |
H2CO3 | K1= 4.3��10 -7��K2= 5.6��10 -11 |
H2SO3 | K1=1.54��10-2 ��K2=1.02��10-7 |
��1��NaHSO3��Һ�й�����7������������Na+��HSO3-��H+��SO32-��H2O��________��_________���������ţ���
��2�������£����ʵ���Ũ����ͬ��������Һ ��
��NH4Cl �� NH4HCO3 �ۣ�NH4��2SO4 �� NH4HSO4
��Һ��c(NH4+)�����ǣ�_________����С���ǣ�______������ţ�
��3�������£����ʵ���Ũ�Ⱦ�Ϊ0��1mol/L��������Һ��NaOH����NaCl����Na2CO3����H2SO3����CH3COONa����H2SO4��pH�Ӵ�С����˳��Ϊ___________������ţ�
��4������ʱ��AlCl3��ˮ��Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ����_____________��AlCl3��Һ���ɣ����գ����õ��Ĺ��������Ҫ��________���ѧʽ��
���𰸡� OH- H2SO3 �� �� �٣��ۣ��ݣ��ڣ��ܣ��� Al3+�� 3H2O ![]() Al (OH)3 �� 3H+ Al2O3
Al (OH)3 �� 3H+ Al2O3
��������(1)NaHSO3��ǿ�������Σ�����ȫ����NaHSO3�TNa++HSO3-��������Һ�д���Na+��HSO3-����Һ�л�����HSO3-����HSO3-H++SO32-��������Һ�д���H+��SO32-��Ҳ����HSO3-ˮ��HSO3-+H2OH2SO3+OH-��������Һ�д���H2SO3��OH-������NaHSO3��Һ�й�����7������������Na+��HSO3-��H+��SO32-��H2O��OH-��H2SO3���ʴ�Ϊ��OH-��H2SO3��
(2)�����£����ʵ���Ũ����ͬ�Ģ�NH4Cl���� NH4HCO3����(NH4)2SO4���� NH4HSO4�Т���c(NH4+)���ԼΪ�����2��������̼���������ˮ��ٽ�笠����ӵ�ˮ�⣬����c(NH4+)��С���ʴ�Ϊ����������
(3)��NaOH����NaCl����Na2CO3����H2SO3����CH3COONa����H2SO4����Ϊǿ�pH���Ϊǿ�ᣬpH��С����Ϊǿ��ǿ���Σ�pH=7���ۢ�Ϊ����ǿ���Σ�ˮ���Լ��ԣ������ԱȢ�������Ϊ���ᣬ����pH�Ӵ�С����˳��Ϊ�����������������������ޣ��ʴ�Ϊ��������������������������
(4)AlCl3Ϊǿ����ʣ�����Һ����ȫ���룬����Al3+���Ӻ�Cl-���ӣ����뷽��ʽΪAlCl3�TAl3++3Cl-��Al3+ˮ�����ӷ���ʽΪ��Al3++3H2OAl(OH)3+3H+��AlCl3��Һ�����ԣ���AlCl3��Һ���ɣ��Ȼ���ӷ����ٽ�ˮ�����������������������յõ����������ʴ�Ϊ��Al3++3H2OAl(OH)3+3H+��Al2O3��


 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йؼ��鼰���ȴ���˵����ȷ����
A.���鼰���ȴ����ж�ֻ���ڼ��Լ�B.���鼰���ȴ��ﶼ����������ṹ
C.���������ȶ��������Ա����Ը��������Һ����D.��������������ȡ����Ӧʱ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����͵��ĵ��ʼ�һЩ�������ڹ�ũҵ��������������ҪӦ�á��ش�����������
��1��Nԭ�Ӻ�����___�ֲ�ͬ�˶�״̬�ĵ��ӡ���̬Nԭ���У�������ߵĵ�����ռ�ݵ�ԭ�ӹ������״Ϊ___________��
��2��Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��һ������ʱ���ų�������������һ�������ܣ�E1�����ڶ����ڲ���Ԫ�ص�E1�仯������ͼ��ʾ�����г���Ԫ���⣬����Ԫ�ص�E1����������������ԭ����_______________________����Ԫ�ص�E1�����쳣��ԭ����_______________________��
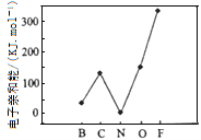
��3�����ⶨ���֣�N2O5������NO2+��NO3-����������ɣ��ù�����Nԭ���ӻ�����Ϊ___________________��
��4����δ��ȶ���NH4F��NH4I�У����ֽ����____��ԭ����__________________��
��5���ڶ������У���һ�����ܽ���BԪ�غ�NԪ�ؼ��Ԫ��Ϊ_____(����Ԫ�ط�����)��
��6����֪����NO2 + CO ![]() CO2 + NO
CO2 + NO
ÿ1mol�������ʷֽ�Ϊ��̬��̬ԭ�����������ֱ�Ϊ
NO2 | CO | CO2 | NO |
812kJ | 1076kJ | 1490kJ | 632kJ |
��N2(g)+O2(g) ![]() 2NO(g) ��H��+179.5 kJ/mol
2NO(g) ��H��+179.5 kJ/mol
��2NO(g) +O2(g)![]() 2NO2(g) ��H��-112.3 kJ/mol
2NO2(g) ��H��-112.3 kJ/mol
��д��NO��CO��Ӧ��������Ⱦ��������Ȼ�ѧ����ʽ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ͼ�жϣ�����������ȷ���ǣ� ��

A. ͼ�����ŵ�ԭ��ع���ʱ��ͭ�缫�Ϸ���������Ӧ��CuSO4��Һ��ɫ����
B. ��͢��и�����Ӧ����Fe��2e��===Fe2��
C. ��͢���������Ӧ����O2��2H2O��4e��===4OH��
D. ��͢��зֱ��������K3[Fe(CN)6]��Һ��������ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������仯��������Ҫ�Ͻ���Ϻʹ������䴢��Ͻ����Ϊһ������п���ӵ�صĸ������ϣ��õ����Zn(CF3SO3)2Ϊ����ʣ�����ȱ�ݵ���������ZnMn2O4Ϊ�缫���ɹ�������ȶ��Ĵ��ʵ�����
��1����̬��ԭ�ӵĺ�������Ų�ʽΪ________�����Ų�ʱ������ߵ�����ռ���ܼ���ԭ�ӹ����________����չ����
��2��VO2+������������γ���������ͬ�����ҵ�һ�����ܱ����������Ԫ����____(дԪ�ط���)��
��3�����γɵ�������[Ni��NH3��6]2+��[Ni(CN)4]2-�У�NH3���ӵĿռ乹��Ϊ_______����CN-��Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽΪ__________��
��4����������(CF3SO3H)��һ���л�ǿ�ᣬ�ṹʽ��ͼ1��ʾ��ͨ����CS2��IF3��H2O2��Ϊ��Ҫԭ������ȡ��
��H2O2������Oԭ�ӵ��ӻ���ʽΪ________��
��������������ⱽ��Ӧ�����������ᱽ���͵⻯�⡣1���������ᱽ�������к�����������ĿΪ__________��

��5����п����Ĺ����ж��֣�����һ����п�ľ�����ͼ2��ʾ���þ�����S2-����λ��Ϊ____��
��6�������Ͻ�����Ҫ������ϣ��䴢���ľ�����ͼ3��ʾ��
�ٴ���ǰ�������Ͻ�Ļ�ѧʽΪ___________��
�ڸ������Ͻ�����������ܶ�Ϊ________(��NA��ʾ�����ӵ���������ֵ)g.cm-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2015��8��12������Σ��Ʒ�ֿⷢ���ı�ը�¹ʣ��ٴξ�ʾ������ע����߿Ƽ�������չˮƽ��ͬʱ�����ɺ��ӻ�ѧ�Լ���ŵĻ������⡣������ʵ�����й����Լ����������ȷ����

A. ���ײ��ӷ�������Ҫ�ܱմ�ţ�
B. �軯��İ�װ��ǩ��Ӧ������ͼ��ʾ�ı�־
C. ����������ҺӦ�����ڴ���������ϸ���Լ�ƿ��
D. ������ƿ���ƺ���Һ��������ʱ����������ƿ������Ũ�ȱ�ǩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100�������֮�ȣ���ʹ�á���ش��������⣺

��1���á�84����Һ�������ʵ���Ũ��ԼΪ___________molL-1��ȡ��������
��2��ijͬѧȡ100mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c��Na+��=_________molL-1��
��3����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480mL��NaClO��������Ϊ25%������Һ������˵����ȷ����________������ţ���
A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ�������

B������ƿ������ˮϴ����Ӧ��ɺ����������Һ����
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
D����Ҫ����NaClO���������Ϊ143.0g
��4����ʵ���������������������Һ�����ʵ���Ũ���ǣ�
A��ƫ�� B��ƫ�� C�����䣨�÷��Żش�
����ʱ���ӿ̶��ߣ�__________��
��δ�������¾�ת�ƶ��ݣ�__________��
��ת��ǰ������ƿ��������ˮ��___________��
��������ʱˮ���ý�ͷ�ι�������____________��
��5����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%���ܶ�Ϊ1.84gcm-3����Ũ��������2000mL2.3molL-1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H+�����ʵ���Ũ��Ϊ________molL-1��
������Ũ��������Ϊ________mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������450 mL3.0 mol��L��1 H2SO4��Һ��ijͬѧ��98%��Ũ���� ���ܶ�Ϊ1.84 g/mL�������в�����������
��1����ش��й����⡣
ʵ�鲽�� | �й����� |
a.��������ŨH2SO4������� | ��ȡŨH2SO4�����Ϊ��__________ |
b.��ȡŨ������ | |
c.��ŨH2SO4�������뵽װ������ˮ��200 mL�ձ����� | |
d.���ձ��е���Һ��ȴ��ת������__________���� | |
e.ϴ�Ӻ�������ƿ�м�����ˮ��̶���1��2cm�����â�__________������ |
��2������H2SO4��Һʱ�����������в���������ƫ����ƫ�ͣ�
A����ȡŨH2SO4��Һʱ���Ӷ��� ____��
B������ʱ���ӿ̶��� ___��
C��Ũ��������ˮ��δ����ȴ��ת��____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com