
ij��ѧ̽��С������SO2�Ļ�ѧ���ʽ�������̽��,������������ʵ�鱨�档
| ���� | ��� | ��ѧ�� ��Ԥ�� | ʵ����֤ | ||
| ʵ����� | ʵ������ | ʵ��(������ ����ʽ��ʾ) | |||
| ���� ���� | ���� ������ | ��ˮ ��Ӧ | ��ʢ��SO2������Թܵ�����ˮ��,���ⶨ�Թ�����Һ��pH | �� | SO2+H2O H2SO3 H2SO3 |
| ��� ��Ӧ | �� | ���ְ� ɫ���� | �� | ||
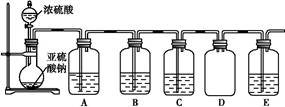
| װ�� | ҩƷ | ���� |
| A | | ��֤��������Ļ�ԭ�� |
| B | | |
| C | Ʒ����Һ | |
(1)���Թ���Һ������,��ҺpH��7
�ڽ�������������ͨ����������ʯ��ˮ[��Ba(OH)2��Һ]��
��SO2+Ca2++2OH-=CaSO3��+H2O(��SO2+Ba2++2OH-=BaSO3��+H2O)
(2)��װ�� ҩƷ ���� A ���Ը��������Һ B Na2S��Һ ��֤��������������� C ��֤���������Ư����
��5SO2+2MnO4-+2H2O=2Mn2++5SO42-+4H+
��Ʒ����Һ��ɫ
�ܷ����� ������,��Ϊ�����ķ�ӦΪ3Ba2++2NO3-+3SO2+2H2O=3BaSO4��+2NO��+4H+,���ɵ�NO��Ȼ�Ի�������Ⱦ
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ʵ�飬ʵ��װ����ͼ��ʾ��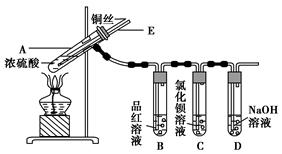
ʵ�鲽�裺
����������ͼ��ʾ��װ�ã����������ԣ��ټ����Լ���
�ڼ���A�Թܣ���B�Թ���Ʒ����Һ��ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿���ϳ鶯�뿪Һ�档
��ش��������⣺
(1)A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)�ܹ�֤��ͭ��Ũ���ᷴӦ���������ʵ�������� ��
(3)��ʢ��BaCl2��Һ��C�Թ��У����˵��ܿ��������⣬���������������������е���Һ�ֳ����ݣ��ֱ�μ�������Һ�������������Ļ�ѧʽ������ж�Ӧ��λ�á�
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ�о���ѧϰС�������ͭ�ֽ��������ijɷֽ�������̽��:
���������ϡ�
����ͭ���ȷֽ���������ͭ������,�¶Ȳ�ͬʱ,�������ΪSO3,SO2��O2�е�һ�֡����ֻ����֡�
��������衿
����1:�������ֻ��һ��;
����2:�������ֻ������;
����3:������������֡�
(1)������1����,���������ijɷ�����������;������2����,���������ijɷ�������������
��ʵ�鼰������ۡ�
(2)��ͬѧ���ֽ����ɵ���������ͨ��ʢŨ�����KMnO4������Һ��ϴ��ƿ,��˵�����������к�SO2�������� ��,������˵��SO2���е���������������,ʵ���л�����ʢŨ�����ϴ��ƿ������������,ԭ������������������ ����
(3)��ͬѧ���ֽ����ɵ�����ͨ����ʯ�Һ�,���ռ��ⶨʣ����������,�ڲ�ͬ�¶��½���3��ʵ�顣������±�(ʵ��������ͭ����ȫ�ֽ�):
| ʵ�� ��� | ��ȡCuSO4 ������/g | ��ʯ�ҵ� ��������/g | ʣ����������(���� �ɱ�״����)/mL | ���� |
| �� | 6.4 | | | ����1���� |
| �� | 6.4 | 2.88 | 224 | |
| �� | 6.4 | 2.56 | 448 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪AgNO3������ȷֽ��������ֵ��ʺͺ���ɫ���塣������ijЩװ�ô��Բⶨ�������������ֽ�Ҳ�����뷴Ӧ�����ʵ��������Ĵ��ȣ��������й�ʵ��(װ���б�Ҫ������̨�����С��ƾ��Ƶ�����ȥ)����д���пհס�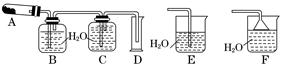
��1��д��AgNO3���ȷֽ�Ļ�ѧ����ʽ��_______________________________________________________________��
��2���ⶨAgNO3�Ĵ��ȣ���ѡ����A��B��C��D��ɵ�װ�ã������в���������____________���ô�������ĺ����____________________________________________��
��3��Bƿ�еĿ�����ʵ����________(��С����ޡ�)Ӱ�죬������____________________________________________________________________________��
��4������Ľ�װ�ú�ȡ����������4.00 g����A�л������ȣ�����Ӧ��ȫ����������ͨ��B��Cװ�ú����Ͳ��ˮ�������������ɱ�״������������Ϊ112 mL���������Ĵ���Ϊ________��
��5���������Cu(NO3)2��������ͭ������ȷֽ���������Ӧͨ��װ��________(�E����F��)����������__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶá���֪��Na2S2O3��������Һ�в����ȶ����ڡ�
��1��ij�о�С����Ƶ��Ʊ�Na2S2O3��5H2O��װ�úͲ��ֲ����������¡�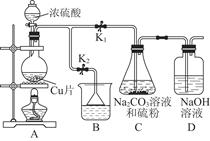
��.��K1�ر�K2����Բ����ƿ�м�������Ũ���ᣬ���ȡ�
��.C�л��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH �ӽ�7ʱ����K2���ر�K1��ֹͣC�еķ�Ӧ��ֹͣ���ȡ�
��.����C�еĻ��Һ��
��.����Һ���� �� �����ˡ�ϴ�ӡ���ɣ��õ���ƷNa2S2O3��5H2O��
�٢��У�����C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ����ԭ���� �������ӷ���ʽ��ʾ����
�ڢ��У����������� �� ��
��װ��B��ʢ�ŵ��Լ��ǣ��ѧʽ�� ��Һ��
����һС����ʵ���з��֣�����������������������º����������ԣ����ֲ��������������⣬�����Ʋ���ܵ�ԭ�� ��
��2������Na2S2O3��Һ�ⶨ��ˮ��Ba2����Ũ�ȣ��������£�ȡ��ˮ25.00 mL�������ʵ�����ȼ������� K2Cr2O7��Һ����BaCrO4���������ˡ�ϴ�Ӻ�������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���ټӹ�KI��Һ����ַ�Ӧ��û����ҺV mL������ƽ���ֳ�4�ȷݣ����������Һ��ָʾ������0.001 0 mol��L��1��Na2S2O3��Һ���еζ�����Ӧ��ȫʱ��������ݼ�¼���±���ʾ��
| ��� | 1 | 2 | 3 | 4 |
| ����Na2S2O3�� | | | | |
| ��Һ�����/mL | 18.02 | 17.98 | 18.00 | 20.03 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ�ϳ�����������ʢװ��Ũ���ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��̽��һ��
��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ����__________________��
��2����ȡ����6.0 g����15.0 mLŨ�����У����ȣ���ַ�Ӧ���ռ�������Y��
��ͬѧȡ336 mL����״��������Yͨ��������ˮ�У�������Ӧ��SO2+Br2+2H2O=2HBr+H2SO4
Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33 g���ɴ���֪����Y��SO2���������Ϊ______��
��̽������
��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��Q���塣Ϊ�����������̽��ʵ��װ�ã�ͼ�мг�����ʡ�ԣ���
��3��װ��B���Լ���������_________________________________________��
��4����Ϊ����Y�л�����Q��������______________________________�����û�ѧ����ʽ��ʾ����
��5��Ϊȷ��Q�Ĵ��ڣ�����װ��������M��______��ѡ����ţ���
a.A֮ǰ b.A��B�� c.B��C�� d.C��D��
��6���������Y�к���H2��Ԥ��ʵ������Ӧ��____________________________��
��7����Ҫ�ⶨ���������Y��H2�ĺ�������״����Լ��28 mL H2���������ò���H2����ķ����⣬�ɷ�ѡ�����������ķ����������жϲ�˵������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ijЩ������Ʊ������ʵ�ʵ��(ͼ�мг�װ����ʡ��)���밴Ҫ����գ�
��.̽�������백���ķ�Ӧ
(1)Ϊ��ȡ���ﰱ�����ɽ�װ��C��________(��װ�ñ��)���ӣ�װ��C�е���ƿ�ڹ�����ѡ��________��
a����ʯ�� b���Ȼ��� c������������ d����ʯ��
(2)װ��A��E��E���ӿ���ȡ�����������������������Eװ���ڵ�ҩƷ������________________��
(3)װ��F������̽�������백��(��֪�����백���ɷ�����Ӧ��3Cl2��2NH3===N2��6HCl)�ķ�Ӧ��ʵ��ʱ���ɼ�1��3���ر�2��������ƿ��ͨ��________��Ȼ��ر�1��3����2������ƿ�л���ͨ��һ��������һ�����塣ʵ��һ��ʱ�����ƿ�ڳ���Ũ��İ��̲��������ڱ����ᣬ�����һ��ʵ�鷽�������ù����е�������______________________________________________________________________________��
��.̽��ijЩ���ʵ�����
(4)����װ��A��E�������ʵ��Ƚ�Cl����Br���Ļ�ԭ��ǿ������֤�����۵�ʵ��������______________________________________________________��
(5)������װ��A��E������ϩ����ˮ��Ӧ��ʵ�飬д����Ӧ�Ļ�ѧ����ʽ________________________________________________________________��
(6)��װ��B��C�ֱ���F��������H2S��SO2��Ӧ��ʵ�顣F����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ______________________��F���ձ������������________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ش�ijͬѧ��̽��Ũ���ᡢϡ���ᡢŨ���ᡢϡ����ֱ���ͭ��Ӧ��ʵ���з��ֵ��й����⡣
��.̽����������������������ǿ��������ͭ��Ӧ�Ļ�ԭ���������
(1)�ֱ���ʢ�е���ͭƬ����֧�Թ��м���������Ũ���ᡢϡ���ᡢŨ���ᡢϡ����,ʵ������¼���±�:
| | �� | ʵ���� |
| a | Ũ���� | ���Ⱥ�����Ӧ,������ɫ�̼������� |
| b | ϡ���� | ����Ҳ��������Ӧ |
| c | Ũ���� | �����ȼ�������Ӧ,��������ɫ���� |
| d | ϡ���� | �ȷ�����Ӧ,������ɫ���� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ�������װ���Ʊ����ռ��������Ȼ�����Ӳ�ʲ�����E��װ��ϸ��˿����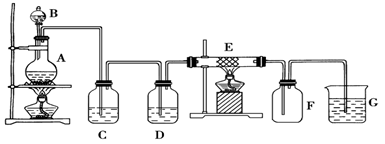
�Իش�
��1������װ��A�������Եķ�����
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ
��3��װ��C�������ǣ� ________________��װ��D�е��Լ��ǣ�____ _______��
��4���ɼ���װ��E�����ɵ������������ӵķ����������� ��
��5����������ͨ��ʯ����Һ�У��۲�������ǣ� ��
��6��װ�� G�з�����Ӧ�����ӷ���ʽΪ��________________ _ ____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com